��Ŀ����
����ʱ�䣹��25��21ʱ10��04�룬�ҹ�������ҵ��ӭ��һ����ʷ��ʱ�̣��ҹ��������Ƶ������ߺ����˷ɴ��ھ�Ȫ���Ƿ������ķ������գ���28������ص������ĵ������ۡ��ߺŻ㼯�˴������¿Ƽ����������ij����ƽ���Ϊ����������ƫ�����£�C2H8N2����5.00g C2H8N2��ȫȼ�տɷų�212.5kJ����������������ȷ���ǣ� ��
| A��ȼ�ղ�һ���������μӣ�Ҳ��һ���Ƿ��ȷ�Ӧ |
| B�������������ĺ�ɫ�����ǽ�������ɫ��Ӧ������ |
| C�����ȼ��ȼ����Ҫ�ǽ���ѧ��ת��Ϊ���ܺ��ܣ����ܶԻ���������Ⱦ |
| D��ƫ������ȼ�յ��Ȼ�ѧ����ʽ�ǣ�C2H8N2(g)+2N2O4(g)=2N2(g)+2CO2(g)+4H2O(g)����H=-2550kJ/mol |
C
ȼ�շ�Ӧ�Ƿ��ⷢ�ȵķ��ȷ�Ӧ������һ���������μӣ����ơ���������������ȼ�գ�A��������������ĺ�ɫ���������������ֽ����ɵĶ���������B��������ȼ��ȼ�պ�ѻ�ѧ��ת��Ϊ���ܺ��ܣ�����ȼ�պ�����л�������������������Ի�������һ������Ⱦ��C����ȷ��������֪C2H8N2ӦΪҺ̬��Ϊ��̬�����Ȼ�ѧ����ʽ��ӦΪC2H8N2��l����D�����

��ϰ��ϵ�д�
 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д� ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�
�����Ŀ
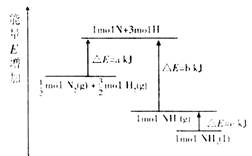
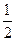 N2(g)+
N2(g)+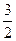 H2(g)=NH3(1); ��H=(b+c-a)kJ��mol-1
H2(g)=NH3(1); ��H=(b+c-a)kJ��mol-1