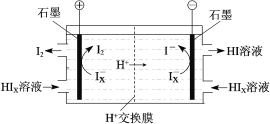
ЬтФПФкШн
ЁОЬтФПЁПСзОЋПѓЪЊЗЈжЦБИСзЫсЕФвЛжжЙЄвеСїГЬШчЯТЃК

вбжЊЃКСзОЋПѓжївЊГЩЗжЮЊCa5(PO4)3(OH)ЃЌЛЙКЌгаCa5(PO4)3FКЭгаЛњЬМЕШЁЃ
ШмНтЖШЃКCa5(PO4)3(OH)<CaSO4ЁЄ0.5H2O
ЃЈ1ЃЉЩЯЪіСїГЬжаФмМгПьЗДгІЫйТЪЕФДыЪЉга__________ЁЃ
ЃЈ2ЃЉСзОЋПѓЗлЫсНўЪБЗЂЩњЗДгІЃК
2Ca5(PO4)3(OH)+3H2O+10H2SO4![]() 10CaSO4ЁЄ0.5H2O+6H3PO4
10CaSO4ЁЄ0.5H2O+6H3PO4
ЂйИУЗДгІЬхЯжГіЫсадЙиЯЕЃКH3PO4__________H2SO4ЃЈЬюЁА>ЁБЛђЁА<ЁБЃЉЁЃ
ЂкНсКЯдЊЫижмЦкТЩНтЪЭЂйжаНсТлЃКPКЭSЕчзгВуЪ§ЯрЭЌЃЌ__________ЁЃ
ЃЈ3ЃЉЫсНўЪБЃЌСзОЋПѓжаCa5(PO4)3FЫљКЌЗњзЊЛЏЮЊHFЃЌВЂНјвЛВНзЊЛЏЮЊSiF4Г§ШЅЁЃаДГіЩњГЩHFЕФЛЏбЇЗНГЬЪНЃК__________ЁЃ
ЃЈ4ЃЉH2O2НЋДжСзЫсжаЕФгаЛњЬМбѕЛЏЮЊCO2ЭбГ§ЃЌЭЌЪБздЩэвВЛсЗЂЩњЗжНтЁЃЯрЭЌЭЖСЯБШЁЂЯрЭЌЗДгІЪБМфЃЌВЛЭЌЮТЖШЯТЕФгаЛњЬМЭбГ§ТЪШчЭМЫљЪОЁЃ80ЁцКѓЭбГ§ТЪБфЛЏЕФдвђЃК____________________ЁЃ

ЃЈ5ЃЉЭбСђЪБЃЌCaCO3ЩдЙ§СПЃЌГфЗжЗДгІКѓШдгаSO42ВаСєЃЌдвђЪЧ__________ЃЛМгШыBaCO3ПЩНјвЛВНЬсИпСђЕФЭбГ§ТЪЃЌЦфРызгЗНГЬЪНЪЧ____________________ЁЃ
ЃЈ6ЃЉШЁa gЫљЕУОЋжЦСзЫсЃЌМгЪЪСПЫЎЯЁЪЭЃЌвдАйРяЯуЗгЬЊзїжИЪОМСЃЌгУb molЁЄL1NaOHШмвКЕЮЖЈжСжеЕуЪБЩњГЩNa2HPO4ЃЌЯћКФNaOHШмвКc mLЃЌОЋжЦСзЫсжаH3PO4ЕФжЪСПЗжЪ§ЪЧ________ЁЃЃЈвбжЊЃКH3PO4ФІЖћжЪСПЮЊ98 gЁЄmol1ЃЉ
ЁОД№АИЁП баФЅЁЂМгШШ ЃМ КЫЕчКЩЪ§PЃМSЃЌдзгАыОЖPЃОSЃЌЕУЕчзгФмСІPЃМSЃЌЗЧН№ЪєадPЃМS 2Ca5(PO4)3F+10H2SO4+5H2O![]() 10CaSO4ЁЄ0.5H2O+6H3PO4+2HFЁќ 80 ЁцКѓЃЌH2O2ЗжНтЫйТЪДѓЃЌХЈЖШЯджјНЕЕЭ CaSO4ЮЂШм BaCO3+
10CaSO4ЁЄ0.5H2O+6H3PO4+2HFЁќ 80 ЁцКѓЃЌH2O2ЗжНтЫйТЪДѓЃЌХЈЖШЯджјНЕЕЭ CaSO4ЮЂШм BaCO3+![]() +2H3PO4
+2H3PO4![]() BaSO4+CO2Ёќ+H2O+2
BaSO4+CO2Ёќ+H2O+2![]()
![]()
ЁОНтЮіЁПЗжЮіЃКСзОЋПѓЗлЫсНўКѓЩњГЩДжСзЫсКЭСзЪЏИрЃЌДжСзЫсОЙ§ЭбгаЛњЬМЁЂЭбСђЕШВНжшЛёЕУОЋжЦСзЫсЁЃ
ЃЈ1ЃЉИљОнЭтНчЬѕМўЖдЛЏбЇЗДгІЫйТЪЕФгАЯьЗжЮіЃЌСїГЬжаФмМгПьЗДгІЫйТЪЕФДыЪЉгаЃКбаФЅЁЂМгШШЁЃ
ЃЈ2ЃЉЂйИљОнЁАЧПЫсжЦШѕЫсЁБЕФИДЗжНтЗДгІЙцТЩЃЌЫсадЃКH3PO4![]() H2SO4ЁЃ
H2SO4ЁЃ
ЂкгУдЊЫижмЦкТЩНтЪЭЃЌPКЭSЕчзгВуЪ§ЯрЭЌЃЌКЫЕчКЩЪ§P![]() SЃЌдзгАыОЖP
SЃЌдзгАыОЖP![]() SЃЌЕУЕчзгФмСІP
SЃЌЕУЕчзгФмСІP![]() SЃЌЗЧН№ЪєадP
SЃЌЗЧН№ЪєадP![]() SЁЃ
SЁЃ
ЃЈ3ЃЉИљОнЁАЧПЫсжЦШѕЫсЁБЕФИДЗжНтЗДгІЙцТЩЃЌCa5ЃЈPO4ЃЉ3FгыH2SO4ЗДгІЩњГЩHFЁЂСзЪЏИрКЭСзЫсЁЃ
ЃЈ4ЃЉЭМЪОЪЧЯрЭЌЭЖСЯБШЁЂЯрЭЌЗДгІЪБМфЃЌВЛЭЌЮТЖШЯТЕФгаЛњЬМЭбГ§ТЪЃЌ80ЁцЧАЮТЖШЩ§ИпЗДгІЫйТЪМгПьЃЌЯрЭЌЪБМфФкгаЛњЬМЭбГ§ТЪдіДѓЃЛ80ЁцКѓЮТЖШЩ§ИпЃЌH2O2ЗжНтЫйТЪДѓЃЌH2O2ХЈЖШЯджјНЕЕЭЃЌЗДгІЫйТЪМѕТ§ЃЌЯрЭЌЬѕМўЯТгаЛњЬМЭбГ§ТЪМѕаЁЁЃ
ЃЈ5ЃЉЭбСђЪБЃЌCaCO3ЩдЙ§СПЃЌГфЗжЗДгІКѓШдгаSO42-ВаСєЃЌдвђЪЧЃКCaSO4ЮЂШмгкЫЎЁЃМгШыBaCO3ПЩНјвЛВНЬсИпСђЕФЭбГ§ТЪЃЌвђЮЊBaSO4ФбШмгкЫЎЃЌЗДгІЕФРызгЗНГЬЪНЮЊBaCO3+SO42-+2H3PO4=BaSO4+CO2Ёќ+2H2PO4-+H2OЁЃ
ЃЈ6ЃЉИљОнЬтвтЙиЯЕЪНЮЊH3PO4~2NaOHЃЌгЩЯћКФЕФNaOHМЦЫуH3PO4ЁЃ
ЯъНтЃКЃЈ1ЃЉбаФЅФмдіДѓЗДгІЮяЕФНгДЅУцЛ§ЃЌМгПьЗДгІЫйТЪЃЌМгШШЃЌЩ§ИпЮТЖШМгПьЗДгІЫйТЪЃЛСїГЬжаФмМгПьЗДгІЫйТЪЕФДыЪЉгаЃКбаФЅЁЂМгШШЁЃ
ЃЈ2ЃЉЂйИљОнЁАЧПЫсжЦШѕЫсЁБЕФИДЗжНтЗДгІЙцТЩЃЌЫсадЃКH3PO4![]() H2SO4ЁЃ
H2SO4ЁЃ
ЂкгУдЊЫижмЦкТЩНтЪЭЫсадЃКH3PO4![]() H2SO4ЃЌPКЭSЕчзгВуЪ§ЯрЭЌЃЌКЫЕчКЩЪ§P
H2SO4ЃЌPКЭSЕчзгВуЪ§ЯрЭЌЃЌКЫЕчКЩЪ§P![]() SЃЌдзгАыОЖP
SЃЌдзгАыОЖP![]() SЃЌЕУЕчзгФмСІP
SЃЌЕУЕчзгФмСІP![]() SЃЌЗЧН№ЪєадP
SЃЌЗЧН№ЪєадP![]() SЁЃ
SЁЃ
ЃЈ3ЃЉИљОнЁАЧПЫсжЦШѕЫсЁБЕФИДЗжНтЗДгІЙцТЩЃЌCa5ЃЈPO4ЃЉ3FгыH2SO4ЗДгІЩњГЩHFЁЂСзЪЏИрКЭСзЫсЃЌЩњГЩHFЕФЛЏбЇЗНГЬЪНЮЊ2Ca5ЃЈPO4ЃЉ3F+10H2SO4+5H2O![]() 10CaSO4ЁЄ0.5H2O+6H3PO4+2HFЁќЁЃ
10CaSO4ЁЄ0.5H2O+6H3PO4+2HFЁќЁЃ
ЃЈ4ЃЉЭМЪОЪЧЯрЭЌЭЖСЯБШЁЂЯрЭЌЗДгІЪБМфЃЌВЛЭЌЮТЖШЯТЕФгаЛњЬМЭбГ§ТЪЃЌ80ЁцЧАЮТЖШЩ§ИпЗДгІЫйТЪМгПьЃЌЯрЭЌЪБМфФкгаЛњЬМЭбГ§ТЪдіДѓЃЛ80ЁцКѓЮТЖШЩ§ИпЃЌH2O2ЗжНтЫйТЪДѓЃЌH2O2ХЈЖШЯджјНЕЕЭЃЌЗДгІЫйТЪМѕТ§ЃЌЯрЭЌЬѕМўЯТгаЛњЬМЭбГ§ТЪМѕаЁЁЃ
ЃЈ5ЃЉЭбСђЪБЃЌCaCO3ЩдЙ§СПЃЌГфЗжЗДгІКѓШдгаSO42-ВаСєЃЌдвђЪЧЃКCaSO4ЮЂШмгкЫЎЁЃМгШыBaCO3ПЩНјвЛВНЬсИпСђЕФЭбГ§ТЪЃЌвђЮЊBaSO4ФбШмгкЫЎЃЌЦфжаSO42-гыBaCO3ЩњГЩИќФбШмЕФBaSO4КЭCO32-ЃЌH3PO4ЕФЫсадЧПгкH2CO3ЃЌдкДжСзЫсжаCO32-зЊЛЏГЩH2OКЭCO2ЃЌЗДгІЕФРызгЗНГЬЪНЮЊBaCO3+SO42-+2H3PO4=BaSO4+CO2Ёќ+2H2PO4-+H2OЁЃ
ЃЈ6ЃЉЕЮЖЈжеЕуЩњГЩNa2HPO4ЃЌдђЯћКФЕФH3PO4гыNaOHЮяжЪЕФСПжЎБШЮЊ1ЃК2ЃЌnЃЈH3PO4ЃЉ=![]() nЃЈNaOHЃЉ=
nЃЈNaOHЃЉ=![]() bmol/L
bmol/L![]() c
c![]() 10-3L=
10-3L=![]() molЃЌmЃЈH3PO4ЃЉ=
molЃЌmЃЈH3PO4ЃЉ=![]() mol
mol![]() 98g/mol=
98g/mol=![]() g=0.049bcgЃЌОЋжЦСзЫсжаH3PO4ЕФжЪСПЗжЪ§ЮЊ
g=0.049bcgЃЌОЋжЦСзЫсжаH3PO4ЕФжЪСПЗжЪ§ЮЊ![]() ЁЃ
ЁЃ

 ПьНнгЂгяжмжмСЗЯЕСаД№АИ
ПьНнгЂгяжмжмСЗЯЕСаД№АИЁОЬтФПЁПLi4Ti5O12КЭLiFePO4ЖМЪЧяЎРызгЕчГиЕФЕчМЋВФСЯЃЌПЩРћгУюбЬњПѓЃЈжївЊГЩЗжЮЊFeTiO3ЃЌЛЙКЌгаЩйСПMgOЁЂSiO2ЕШдгжЪЃЉРДжЦБИЃЌЙЄвеСїГЬШчЯТЃК
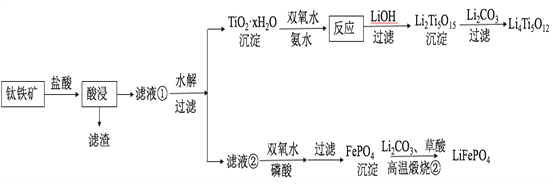
ЛиД№ЯТСаЮЪЬтЃК
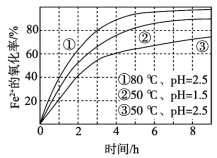
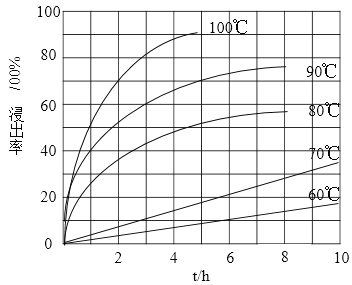
ЃЈ1ЃЉЁАЫсНўЁБЪЕбщжаЃЌЬњЕФНўГіТЪНсЙћШчЯТЭМЫљЪОЁЃгЩЭМПЩжЊЃЌЕБЬњЕФОЛГіТЪЮЊ70%ЪБЃЌЫљВЩгУЕФЪЕбщЬѕМўЮЊ___________________ЁЃ
ЃЈ2ЃЉЁАЫсНўЁБКѓЃЌюбжївЊвдTiOCl42ЃаЮЪНДцдкЃЌаДГіЯргІЗДгІЕФРызгЗНГЬЪН__________________ЁЃ
ЃЈ3ЃЉTiO2ЁЄxH2OГСЕэгыЫЋбѕЫЎЁЂАБЫЎЗДгІ40 minЫљЕУЪЕбщНсЙћШчЯТБэЫљЪОЃК
ЮТЖШ/Ёц | 30 | 35 | 40 | 45 | 50 |
TiO2ЁЄxH2OзЊЛЏТЪ% | 92 | 95 | 97 | 93 | 88 |
ЗжЮі40ЁцЪБTiO2ЁЄxH2OзЊЛЏТЪзюИпЕФдвђ__________________ЁЃ
ЃЈ4ЃЉLi2Ti5O15жаTiЕФЛЏКЯМлЮЊ+4ЃЌЦфжаЙ§бѕМќЕФЪ§ФПЮЊ__________________ЁЃ
ЃЈ5ЃЉШєЁАТЫвКЂкЁБжаc(Mg2+)=0.02 mol/LЃЌМгШыЫЋбѕЫЎКЭСзЫсЃЈЩшШмвКЬхЛ§діМг1БЖЃЉЃЌЪЙFe3+ЧЁКУГСЕэЭъШЋМДШмвКжаc(Fe3+)=1ЁС10-5 mol/LЃЌДЫЪБЪЧЗёгаMg3(PO4)2ГСЕэЩњГЩЃП___________ЃЈСаЪНМЦЫуЃЉЁЃ
FePO4ЁЂMg3(PO4)2ЕФKspЗжБ№ЮЊ1.3ЁС10-22ЁЂ1.0ЁС10-24ЁЃ
ЃЈ6ЃЉаДГіЁАИпЮТьбЩеЂкЁБжагЩFePO4жЦБИLiFePO4ЕФЛЏбЇЗНГЬЪН______ЁЃ
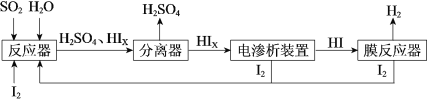
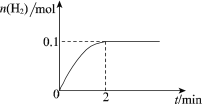
ЁОЬтФПЁПНижЙ2020Фъ4дТ5ШеЃЌШЋЧђаТаЭЙкзДВЁЖОЗЮбзШЗеяГЌЙ§120ЭђР§ЁЃвдЯТЖддЄЗРаТЙкВЁЖОЕФШЯЪЖЃЌВЛЗћКЯПЦбЇЕРРэЕФЪЧЃЈ ЃЉ
A | B | C | D |
|
|
|
|
84ЯћЖОвКЪЙгУЪБВЛФмКЭНрВоСщЛьгУ | вНгУОЦОЋЯћЖОаЇЙћХЈЖШ95%>75% | ПкежЙиМќвЛВуОлБћЯЉШлХчВМЪєгкгаЛњИпЗжзгВФСЯ | ЮТЖШМЦжаЫЎвјЪєгкН№ЪєЕЅжЪ |
A.AB.BC.CD.D