��Ŀ����
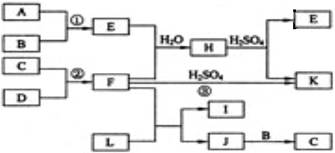
(10��)����ͼ��ʾ�ķ�Ӧ��ϵ��A����ѧ���������A��B��C�к���ͬһ��Ԫ��R������R��Ԫ���Ѿ���ȥ��
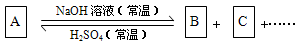
(1)��A��NaOH����ͬ���ʵ����ȷ�Ӧ���ȿ�ֻ����B���ֿ�ֻ����C����������B+C��
��д������������Ӧ��ϵ��A�Ļ�ѧʽ ��
��д�������йط�Ӧ�����ӷ���ʽ��
����B�����ӷ���ʽΪ ��
����C�����ӷ���ʽΪ ��
��2����A��NaOH�����Ժ������ʵ����ȷ�Ӧ��������ΪB+C����A�ķ���ʽ������
��ֻдһ�֣����÷�Ӧ�����ӷ���ʽΪ ��
R��A��B��C�еĻ��ϼ۱�������������� ��

(1)��A��NaOH����ͬ���ʵ����ȷ�Ӧ���ȿ�ֻ����B���ֿ�ֻ����C����������B+C��
��д������������Ӧ��ϵ��A�Ļ�ѧʽ ��
��д�������йط�Ӧ�����ӷ���ʽ��
����B�����ӷ���ʽΪ ��
����C�����ӷ���ʽΪ ��
��2����A��NaOH�����Ժ������ʵ����ȷ�Ӧ��������ΪB+C����A�ķ���ʽ������
��ֻдһ�֣����÷�Ӧ�����ӷ���ʽΪ ��
R��A��B��C�еĻ��ϼ۱�������������� ��
��10�֣�
CO2����AlCl3���� CO2+2OH-=CO32-+H2O�� CO2+OH-=HCO3-��
Cl2+2OH-=Cl-+ClO-+H2O�� R��A�еĻ��ϼ۴���B��C�л��ϼ�֮�䣻
CO2����AlCl3���� CO2+2OH-=CO32-+H2O�� CO2+OH-=HCO3-��
Cl2+2OH-=Cl-+ClO-+H2O�� R��A�еĻ��ϼ۴���B��C�л��ϼ�֮�䣻
��

��ϰ��ϵ�д�
 ������ʱͬ����ϰ��ϵ�д�
������ʱͬ����ϰ��ϵ�д�
�����Ŀ
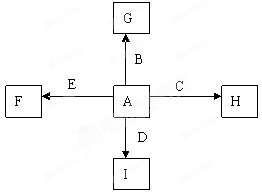
 ��ɫ
��ɫ �� ����Ԫ�ؾ�Ϊ��ϡ������Ԫ�ء����й���������Ԫ�ؼ��仯�����˵������ȷ����( )
�� ����Ԫ�ؾ�Ϊ��ϡ������Ԫ�ء����й���������Ԫ�ؼ��仯�����˵������ȷ����( )