��Ŀ����
13���±��еġ�ʵ����ۡ����Ӧ�ġ�ʵ�������������ȫ����ϵ�һ���ǣ�������| ʵ����������� | ʵ����� | |
| A | ��SO2ͨ��Ʒ����Һ�У���ɫ����ȥ���ټ�������ɫ����Һ����Һ�ָֻ�Ϊ��ɫ | ���ɵ�������ȶ� |
| B | ��ij����Һ�м���ŨNaOH��Һ�����ȣ�����������ʹʪ��ĺ�ɫʯ����ֽ����ɫ | ԭ��Һ�к���NH+4 |
| C | ��ij��������Һ�м�������ϡH2SO4���ữ���ٵμӼ���KSCN��Һ����Һ���ɫ | ԭ������ΪFe��NO3��3 |
| D | ������Һ��ϡ�������ȣ�һ��ʱ������������Һ����ˮ�ܼ��ȣ��Թ��ڱ�δ�������� | ����һ����δˮ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
���� A������������Ʒ�컯��������ɫ���ʲ��ȶ���
B������������ɰ�����������ʹʪ��ĺ�ɫʯ����ֽ����ɫ��
C����������������Һ�о���ǿ�����ԣ��������������ӣ�
D��ˮ��������ԣ�����ˮ�����Ӧ�ڼ��������£�
��� �⣺A������������Ʒ�컯��������ɫ���ʲ��ȶ�����Һ��ɫ����������ɫ����Һ����Һ�ָֻ�Ϊ��ɫ����������IJ��ȶ��أ���A����
B������������ɰ�����������ʹʪ��ĺ�ɫʯ����ֽ����ɫ����ԭ��Һ�к���NH4+����B��ȷ��
C����������������Һ�о���ǿ�����ԣ��������������ӣ�Ȼ���ٵμӼ���KSCN��Һ����Һ���ɫ����ԭ������ΪFe��NO3��2����C����
D��ˮ��������ԣ�����ˮ�����Ӧ�ڼ��������£���ˮ���ֱ�Ӽ�������Һ���ܼ���ˮ��������ȷ��ˮ��̶ȣ���D����
��ѡB��
���� ���⿼�黯ѧʵ�鷽�������ۣ�Ϊ�߿��������ͣ��漰���ʵ����ʡ�������Ʊ������Ӽ����������ԭ��Ӧ���л�������ʼ�����ȣ��������ʵ����ʡ���Ӧԭ��Ϊ���Ĺؼ������ط�����ʵ�������Ŀ��飬ע��ʵ��������Է�������Ŀ�ѶȲ���

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
8������˵��������ǣ�������
| A�� | ʵ������ȡ����ˮ���ú�ˮɹ�ζ���������ͬ�����ʷ��뷽�� | |
| B�� | ��ʢ������������Һ���Թ��м���һ����ϡ���ᣬ����3��4���Ӻ��ټ���NaOH��Һ�����ԣ�������������Һ��ˮԡ���ȣ��С����������� | |
| C�� | ��ǿ�ʴ����ʱ��Ӧ���ô���ˮ��ϴ������2%�Ĵ�����Һ��������Һϴ�������ˮ��ϴ�������������һ������ | |
| D�� | ���ñ�ɫ�ķ����ⶨ��Һ��ɫ����dz��������Һ��ɫ�뷴Ӧ��Ũ�ȵĹ�ϵ���ɻ���ɷ�Ӧ���ڲ�ͬ��Ӧʱ�̵�Ũ�� |
5����ԭ��M�ܲ㺬�еĹ����Ϊ��������
| A�� | 4 | B�� | 5 | C�� | 7 | D�� | 9 |
2�� ������Ԫ�ؼס��ҡ����������졢�����������ڱ��е����λ����ͼ���ײ�һ���ڶ������������ϣ����졢���ֱ��ǿ������ؿ��к�������Ԫ�أ������ж���ȷ���ǣ�������
������Ԫ�ؼס��ҡ����������졢�����������ڱ��е����λ����ͼ���ײ�һ���ڶ������������ϣ����졢���ֱ��ǿ������ؿ��к�������Ԫ�أ������ж���ȷ���ǣ�������
 ������Ԫ�ؼס��ҡ����������졢�����������ڱ��е����λ����ͼ���ײ�һ���ڶ������������ϣ����졢���ֱ��ǿ������ؿ��к�������Ԫ�أ������ж���ȷ���ǣ�������
������Ԫ�ؼס��ҡ����������졢�����������ڱ��е����λ����ͼ���ײ�һ���ڶ������������ϣ����졢���ֱ��ǿ������ؿ��к�������Ԫ�أ������ж���ȷ���ǣ�������| A�� | ��һ���ǽ���Ԫ�� | |
| B�� | ��̬�⻯����ȶ��ԣ����������� | |
| C�� | ��������������ˮ����������ǿ | |
| D�� | �ҡ�������������������ˮ������������Ӧ |
3��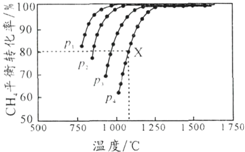 ���ܱ������г������ʵ���Ũ�Ⱦ�Ϊ 0.1mol/L��CH4�� CO2����һ�������·�����ӦCH4��g��+CO2��g��?2CO��g��+2H2��g�������CH4��ƽ��ת�������¶ȼ�ѹǿ�Ĺ�ϵ��ͼ��ʾ������˵������ȷ���ǣ�������
���ܱ������г������ʵ���Ũ�Ⱦ�Ϊ 0.1mol/L��CH4�� CO2����һ�������·�����ӦCH4��g��+CO2��g��?2CO��g��+2H2��g�������CH4��ƽ��ת�������¶ȼ�ѹǿ�Ĺ�ϵ��ͼ��ʾ������˵������ȷ���ǣ�������
 ���ܱ������г������ʵ���Ũ�Ⱦ�Ϊ 0.1mol/L��CH4�� CO2����һ�������·�����ӦCH4��g��+CO2��g��?2CO��g��+2H2��g�������CH4��ƽ��ת�������¶ȼ�ѹǿ�Ĺ�ϵ��ͼ��ʾ������˵������ȷ���ǣ�������
���ܱ������г������ʵ���Ũ�Ⱦ�Ϊ 0.1mol/L��CH4�� CO2����һ�������·�����ӦCH4��g��+CO2��g��?2CO��g��+2H2��g�������CH4��ƽ��ת�������¶ȼ�ѹǿ�Ĺ�ϵ��ͼ��ʾ������˵������ȷ���ǣ�������| A�� | ƽ��ʱCO��H2�����ʵ�����Ϊ1��1 | |
| B�� | p1��p2��p3��p4��С�����˳��Ϊp1��p2��p3��p4 | |
| C�� | 1100�棬p4�����£��÷�Ӧ10 minʱ�ﵽƽ���X����ͣ�CO2��=0.008 mol•L-1•min-1 | |
| D�� | �����¶����ߣ��÷�Ӧ��ƽ�ⳣ����С |
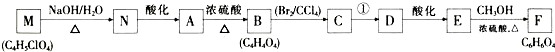
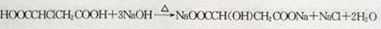 ��
�� ��
�� X��Y��Z��W��Q��ԭ���������������ǰ������Ԫ�أ���֪X��Y��Z��W��Ϊ�ǽ���Ԫ�أ�X�Ļ�̬ԭ����2��δ�ɶԵ��ӣ�XW2������Y3Ϊ�ȵ����壬Ԫ��W��ԭ����������Ԫ��Z��ԭ��������8��Q������������Ϊ2����������������Y��W2-����������֮�ͣ�����������Ϣ�ش��������⣺
X��Y��Z��W��Q��ԭ���������������ǰ������Ԫ�أ���֪X��Y��Z��W��Ϊ�ǽ���Ԫ�أ�X�Ļ�̬ԭ����2��δ�ɶԵ��ӣ�XW2������Y3Ϊ�ȵ����壬Ԫ��W��ԭ����������Ԫ��Z��ԭ��������8��Q������������Ϊ2����������������Y��W2-����������֮�ͣ�����������Ϣ�ش��������⣺ ��
��