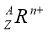��Ŀ����
��Ҫ��д�����з���ʽ��
��1��������Ͷ��FeCl3��Һ�з�Ӧ�������ӷ���ʽ ��
��2����������Ͷ������ͭ��Һ�������ӷ���ʽ ��
��3��̼���Ⱶ�������������Ʒ�Ӧ�����ӻ�ѧ����ʽ ��
��4��������̼�����Ʒ�Ӧ�����ӷ���ʽ_____________________________________________��
��5��������������������Һ��Ӧ�����ӷ���ʽ_______________________________________��
��6����NaHSO4��Һ��������������Һ�����ԣ�������Ӧ�����Һ�е����������������ӷ���ʽ
��
��1��6Na+6H2O+2Fe3+��6Na++2Fe(OH)3+3H2�� ��2��2Na2O2+2H2O+2Cu2+��O2��+2Cu(OH)2+4Na+
��3��Ba2++2HCO3-+2OH-��BaCO3��+CO32-+2H2O ��4��CH3COOH+HCO3-��CH3COO-+H2O+CO2��
��5��Na2O+2H+��2Na++H2O ��6��Ba2++2H++SO42-+2OH-��BaSO4��+2H2O Ba2++SO42-��BaSO4��
��������
�����������1�����ǻ��õĽ���������ˮ��Ӧ�����������ƺ����������ɵ����������ٺ���Һ�е����ʷ������ֽⷴӦ������������Ͷ��FeCl3��Һ�з�Ӧ�������ӷ���ʽΪ6Na��6H2O��2Fe3+��6Na+��2Fe(OH)3��3H2����
��2����������Ͷ������ͭ��Һ�й�������������ˮ��Ӧ�����������ƺ����������ɵ��������ƺ�����ͭ��Ӧ����������ͭ��ɫ�����������ƣ���������ӷ���ʽΪ2Na2O2��2H2O��2Cu2+��O2����2Cu(OH)2��4Na+��
��3��̼���Ⱶ�������������Ʒ�Ӧ����̼�ᱵ��ɫ������̼���ƺ�ˮ����Ӧ�����ӷ���ʽΪBa2+��2HCO3-��2OH-��BaCO3����CO32-��2H2O��
��4�����������ᣬӦ���û�ѧʽ��ʾ����������̼�����Ʒ�Ӧ�����ӷ���ʽΪCH3COOH+HCO3-��CH3COO-+H2O+CO2����
��5��������������������Һ��Ӧ���������ƺ�ˮ����Ӧ�����ӷ���ʽΪNa2O+2H+��2Na++H2O��
��6����NaHSO4��Һ��������������Һ����������ʱ�����������ᱵ�������ƺ�ˮ����Ӧ�����ӷ���ʽΪBa2++2H++SO42-+2OH-��BaSO4��+2H2O��������Ӧ�����Һ�е��������������������Ƽ���������������Ӧ�������ᱵ��ɫ���������Է�Ӧ�����ӷ���ʽΪBa2++SO42-��BaSO4����
���㣺�������ӷ���ʽ����д