��Ŀ����
����Ŀ����ʵ������������230mL 0.1mol/L��Na2CO3��Һ����ղ���ش��������⣺
��1��ʵ����������ƽ��Na2CO3����______________��
��2��Ӧѡ������ƿ�Ĺ��Ϊ��_________��������ƿ���Ҫ������������______��
��3������ʱ������ȷ�IJ���˳���ǣ���ĸ��ʾ��ÿ����ĸֻ����һ�Σ�___________��
A����30mLˮϴ���ձ�2~3�Σ�ϴ��Һ��ע������ƿ����
B����������ƽȷ����ȡ�����Na2CO3�������������ձ��У��ټ�������ˮ��Լ30mL�����ò���������������ʹ����ȫ�ܽ�
C��������ȴ��Na2CO3��Һ�ز�����ע��һ����������ƿ��
D��������ƿ�ǽ�����ҡ��
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ����ǡ����̶�����
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�1~2cm��
��4�������������������������ҺŨ�Ƚ��к�Ӱ�죨����ƫ��������ƫ����������Ӱ������
����û�н���A����_______________��
����������ˮʱ���������˿̶�_______________��
��ʵ����������250 mL 0.1 mol��L�Ĵ��ᣨCH3COOH��������36%�Ĵ��ᣬ�ܶ�Ϊ1.04g��mL����Ҫ��ش��������⣺
��1����Ҫ��Ͳȡ��36%�Ĵ���________ mL�������ƣ�
��2��������ʱ��������һ����������ͼ��ʾ���д�����������ȫ����ȷ������������Ũ��___________��������ȷ������ƫ��������ƫ��������
���𰸡�2.7g 250mL ������ƽ���ձ�������������ͷ�ι� B��C��A��F��E��D ƫ�� ƫ�� 4.0 ƫ��
��������
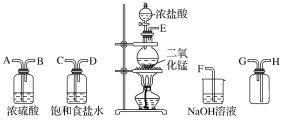
��������230mL 0.1mol/L��Na2CO3��Һ��Ӧѡ��250mL������ƿ��ʵ�鲽�裬���㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȣ�
��������c=![]() ����Ũ�����Ũ�ȣ�����c1��V1=c2��V2���������
����Ũ�����Ũ�ȣ�����c1��V1=c2��V2���������
������1������230mL 0.1mol/L��Na2CO3��Һ��Ӧѡ��250mL������ƿ������250mL����Һ�������Na2CO3����=c��V��M=0.1mol/L��0.25L��106g/mol=2.65g����Ҫ����2.7g��
��2��Ӧѡ������ƿ�Ĺ��Ϊ250mL������ʵ�鲽�裬���㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȣ�ʹ�õ�������:��ƽ����Ͳ���ɲ�ѡ�����ձ�����������250mL������ƿ����ͷ�ιܣ���Ϊ������ƽ���ձ�������������ͷ�ιܣ�
��3������ʱ������ʵ�鲽�裬���㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȣ������˳��ΪB��C��A��F��E��D��
��4������û�н���ϴ�Ӳ���������������Һ�����ʵ����ʵ���ƫС����Ũ��ƫ�ͣ�
����������ˮʱ���������˿̶ȣ�������Һ�����ƫ��Ũ��ƫ�ͣ�
������1��36%�Ĵ����Ũ��=![]() =
=![]() =6.24mol/L��V��CH3COOH��=
=6.24mol/L��V��CH3COOH��=![]() mL=4.0mL��
mL=4.0mL��
��2������ͼ���֪������ʱ���ӣ�����������Һ�����ƫ������ҺŨ��ƫ�͡�

����Ŀ������������������OH-�����·���ˮ�ⷴӦ��O2NC6H4COOC2H5+OH-O2NC6H4COOO-+C2H5OH���ַ�Ӧ��ij�ʼŨ�Ⱦ�Ϊ0.050mol/L���ڲ�ͬ�¶��²��O2NC6H4COOC2H5��Ũ�ȣ�mol/L����ʱ��仯���������±���ʾ�������й�˵������ȷ���ǣ�������
t/s | 0 | 120 | 180 | 240 | 330 | 530 | 600 | 700 | 800 |
15�� | 0.500 | 0.335 | 0.291 | 0.256 | 0.210 | 0.155 | 0.148 | 0.145 | 0.145 |
35�� | 0.500 | 0.325 | 0.2775 | 0.238 | 0.190 | �� | 0.135 | 0.135 | 0.135 |
A. �÷�Ӧ��![]() ��120180s������O2NC6H4COOC2H5ƽ����Ӧ����Ϊ7.33��10 -4 mol��L-1��s-1
��120180s������O2NC6H4COOC2H5ƽ����Ӧ����Ϊ7.33��10 -4 mol��L-1��s-1
B. �����ݿ�֪�����ŷ�Ӧ�Ľ��У���Ӧ���Ũ�Ƚ��ͣ���Ӧ���ʼ���
C. 530sʱ��������35���Ӧ������һ����0.135
D. �����ݿ�֪���¶����߷�Ӧ���ʼӿ�
����Ŀ���������ӷ���ʽ����д�����ۣ�����������
ѡ�� | ���ӷ���ʽ | ���� |
A | ��ͭ�缫��ⱥ��KCl��Һ��2H2O+2Cl- | ��ȷ��Cl-��ʧ����������OH-ǿ |
B | ��CuSO4��Һ��ͨ�������H2S���壺Cu2++H2S=CuS��+2H+ | ����H2S�����Ա�H2SO4�� |
C | Ba(HCO3)2��Һ��������NaOH��Һ��Ӧ��Ba2++HCO3- +OH- �TBaCO3��+H2O | ����Ba2+��HCO3-ϵ����ӦΪ1:2 |
D | ����SO2ͨ�뵽NaClO��Һ�У�SO2+ClO- +H2O= HClO+HSO3- | ��ȷ��H2SO3�����Ա�HClOǿ |
A.AB.BC.CD.D