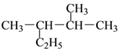��Ŀ����
����Ŀ����NA��ʾ�����ӵ�������ֵ������˵������ȷ����Ŀ��
��12.0g���ڵ�NaHSO4�к��е���������Ϊ0.2NA
��1mol Na2O ��Na2O2�����������������������3NA
�۳��³�ѹ�£�92g��NO2��N2O4������庬�е�ԭ����Ϊ6NA
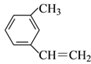
��7.8g![]() �к��е�̼̼˫����ĿΪ0.3NA
�к��е�̼̼˫����ĿΪ0.3NA
����1L1.0 mol/LFeCl3��Һ�Ʊ������������壬��������������������ĿΪNA
��1mol SO2������O2��һ�������³�ַ�Ӧ����SO3����ת��2NA������
���ڷ�ӦKIO3+6HI=KI+3I2 +3H2O �У�ÿ����3molI2ת�Ƶĵ�����Ϊ5NA
�ೣ�³�ѹ�£�17 g��(��14CH3)��������������Ϊ9NA
A. 3 B. 4 C. 5 D. 6
���𰸡�A
����������n��NaHSO4��=![]() =0.1mol��NaHSO4������״̬�µĵ��뷽��ʽΪNaHSO4=Na++HSO4-��12.0g���ڵ�NaHSO4�к��е����������ʵ���Ϊ0.1mol������������Na2O��Na2O2�����������Ӹ���֮�ȶ�Ϊ1:2��1molNa2O��Na2O2������к��е����������������ʵ���Ϊ3mol������ȷ����NO2��N2O4��ʵ��ʽ����NO2��n��NO2��=
=0.1mol��NaHSO4������״̬�µĵ��뷽��ʽΪNaHSO4=Na++HSO4-��12.0g���ڵ�NaHSO4�к��е����������ʵ���Ϊ0.1mol������������Na2O��Na2O2�����������Ӹ���֮�ȶ�Ϊ1:2��1molNa2O��Na2O2������к��е����������������ʵ���Ϊ3mol������ȷ����NO2��N2O4��ʵ��ʽ����NO2��n��NO2��=![]() =2mol�����³�ѹ��92g��NO2��N2O4�������������ԭ�����ʵ���Ϊ6mol������ȷ�������в���̼̼˫��������������n��FeCl3��=1.0mol/L
=2mol�����³�ѹ��92g��NO2��N2O4�������������ԭ�����ʵ���Ϊ6mol������ȷ�������в���̼̼˫��������������n��FeCl3��=1.0mol/L![]() 1L=1mol�����ݷ�ӦFeCl3+3H2O
1L=1mol�����ݷ�ӦFeCl3+3H2O![]() Fe��OH��3�����壩+3HCl������1molFe��OH��3����������������һ����ĿFe��OH��3�ļ����壬�����������������ʵ���С��1mol��������������1molSO2ȫ����Ӧ��ת��2mol��������SO2��O2�ķ�Ӧ�ǿ��淴Ӧ��1molSO2������O2��һ�������³�ַ�Ӧ����SO3��ת�Ƶ������ʵ���С��2mol��������������˫���ŷ����÷�Ӧ��
Fe��OH��3�����壩+3HCl������1molFe��OH��3����������������һ����ĿFe��OH��3�ļ����壬�����������������ʵ���С��1mol��������������1molSO2ȫ����Ӧ��ת��2mol��������SO2��O2�ķ�Ӧ�ǿ��淴Ӧ��1molSO2������O2��һ�������³�ַ�Ӧ����SO3��ת�Ƶ������ʵ���С��2mol��������������˫���ŷ����÷�Ӧ��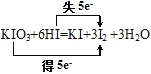 ��ÿ����3molI2ת��5mol����������ȷ����n��-14CH3��=
��ÿ����3molI2ת��5mol����������ȷ����n��-14CH3��=![]() =1mol�������������ʵ���Ϊ8mol������������ȷ�����ڢۢ�����ѡA��
=1mol�������������ʵ���Ϊ8mol������������ȷ�����ڢۢ�����ѡA��

����Ŀ��ij��ѧС���Ա�����Ϊԭ�ϣ���ȡ�������������֪�й����ʵķе����±���
���� | �״� | ������ | ��������� |
�е㣯�� | 64.7 | 249 | 199.6 |
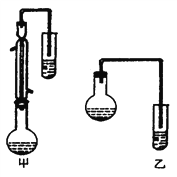
I���ϳɱ���������ֲ�Ʒ
��Բ����ƿ�м���12.2g �������20 mL �״����ܶ�Լ0.79g �� mL��1) ����С�ļ���3 mL Ũ���ᣬ���Ⱥ�Ͷ�뼸�����Ƭ��С�ļ���ʹ��Ӧ��ȫ���ñ���������ֲ�Ʒ��
(1)Ũ�����������_________������Ӧ����ˮ��������ͬλ��18O��д���ܱ�ʾ��Ӧǰ��18Oλ�õĻ�ѧ����ʽ��__________________��
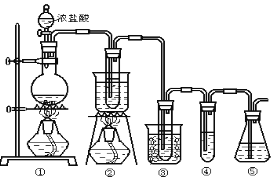
(2)������λͬѧ�ֱ����������ͼ����ʵ���Һϳɱ����������װ�ã��г������ͼ�������������ȥ���������л���ķе㣬��ò���_________װ�ã���ס����ҡ�����������___________________��
(3)��Ӧ��CH3 OH Ӧ������������__________________________________��
II���ֲ�Ʒ�ľ���
(4)����������ֲ�Ʒ���������������״������ᡢ�������ˮ�ȣ��ְ���������ͼ���о��ƣ���������ͼ��������������ǡ����������������______________��
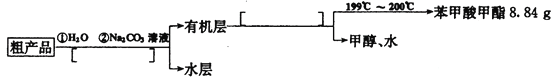
(5)ͨ�����㣬����������IJ���Ϊ_________________________��