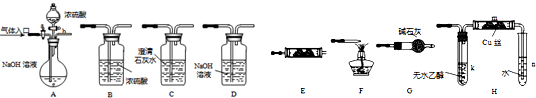
��Ŀ����
((A)��(B)Ϊѡ���⣬������ѡһ������������ͬʱ���𣬰�(A)����)(A)�л���ѧ��Ӧ��Ӧ������ͬ�������ɲ�ͬ���л���Ʒ�����磺

����ͬϵ����±�ص��ʻ�ϣ����ڹ��������£���������ԭ�ӱ�±��ԭ��ȡ�������ڴ��������£������ϵ���ԭ�ӱ�±��ԭ��ȡ����
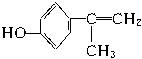
��ҵ������������Ϣ��������·�ߺϳɽṹ��ʽΪ �����ʣ���������һ�����ϡ�
�����ʣ���������һ�����ϡ�

���������·�ߣ��ش��������⣺
(1)A�Ľṹ��ʽ����Ϊ________________________��
(2)��Ӧ�ۢݵķ�Ӧ���ͷֱ�Ϊ________________________��_____________________��
(3)��Ӧ�ܵĻ�ѧ����ʽΪ(�л���д�ṹ��ʽ����ע����Ӧ����)��_______________��
(4)��ҵ�����У��м����A�뾭��Ӧ�ۢܢݵ�D��������ȡֱ��ת��ΪD�ķ�������ԭ����____________________________________________________________________��
(5)�������Ͼ��ж���ͬ���칹�壬����ijЩ��������������������ˮ��Һ��FeCl3��Һ����ɫ���ڷ������б������ұ����ϵ�һ����������֡�д�������������������ʿ��ܵĽṹ��ʽ(ֻд����)��___________________________________��
28.(B)A��B��C��D��E���Ƕ�����Ԫ�أ�ԭ��������������B��Cͬ���ڣ�C�ķǽ�������ǿ��A��Dͬ��������ڣ�EԪ��ԭ��������p�Dz��������s�Dz��������һ�롣A��B���γ�����Һ̬��������ң�ԭ�Ӹ����ȷֱ�Ϊ2��1��1��1������������Ϣ�ش��������⣺
(1)�ס����������к��зǼ��Թ��ۼ������ʵĵ���ʽ��________________________��CԪ�������ڱ��е�λ����___________________________��
(2)C��D�������У��뾶��С����_____________(�����ӷ���)��
(3)��D�ĵ���Ͷ����У���D��ʧ������������Һ�м���E�ĵ��ʣ���ʱ������Ӧ�Ļ�ѧ����ʽ��___________________________________________________________________��
(4)C��D��E��������ӻ�����侧��(�������ھ����о��д����Ե���С�ظ���Ԫ)�ṹ����ͼ��ʾ��������D��(��![]() ��ʾ)λ�������������е���������ڲ���������
��ʾ)λ�������������е���������ڲ���������![]() (��
(��![]() ��ʾ)λ�ڸ�������Ķ�������ġ��û�����Ļ�ѧʽ��________________��
��ʾ)λ�ڸ�������Ķ�������ġ��û�����Ļ�ѧʽ��________________��

(5)��ͼ�����Цġ��á�������ͬ�������壬���־����ڲ�ͬ�¶����ܷ���ת��������˵����ȷ����( )
A.��-Fe��������ÿ����ԭ�Ӿ���������������ԭ����6��
B.��-Fe��������ÿ����ԭ�Ӿ���������������ԭ����6��
C.����-Fe�����߳�Ϊa cm����-Fe�����߳�Ϊb cm�������־����ܶȱ�Ϊ2b3��a3
D.�������ȵ�1

��A��
(1)
(2)��ȥ ˮ���ȡ��
(3)
(4) ��ˮ�����
��ˮ����� ���ܾ�������Ӧ���õ���Ʒ(��A��
���ܾ�������Ӧ���õ���Ʒ(��A�� ��ˮ�����
��ˮ����� �еġ�OH�������ˣ����������ɡ�CHO)(���������𰸾���)
�еġ�OH�������ˣ����������ɡ�CHO)(���������𰸾���)
(5)

(��![]() ��
�� )
)
������(A)����Ϊ�л��ϳ��⣬ע��ϳ��������ʡ�
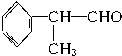 ���������Ƴ���(1)(2)(3)���������
���������Ƴ���(1)(2)(3)���������
(4)��A��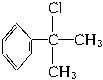 ��ˮ�����
��ˮ�����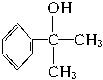 �����ܱ���������
�����ܱ���������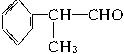 ��
��
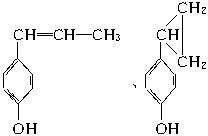
(5)���ܵĽṹ�У�
 ��
�� ��
��
��B��(1)![]() �ڶ����ڡ��ڢ�A��
�ڶ����ڡ��ڢ�A��
(2)Na+
(3)2Al��2NaOH��2H2O![]() 2NaAlO2��3H2��
2NaAlO2��3H2��
(4)Na3AlF6
(5)BC
������(B)������A��B���γ�����Һ̬���������ԭ�Ӹ�����Ϊ2��1��1��1֪����H2O������H2O2���Ӷ�A��H��B��O��D��Na��C��F��E��Al����(1)(2)(3)�ɺ����ó��𰸡�
(4)n(Na+)��n(![]() )=(1+
)=(1+![]() ��4)��
��4)��![]() ��4=3��1��
��4=3��1��
���Ի�ѧʽΪNa3AlF6��

 ��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
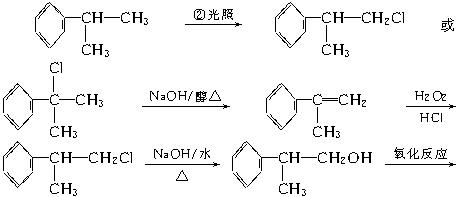
��ĩ���䵥Ԫ�����ิϰ��ϵ�д�1.���л���ѧ�������ұص���һ�ָ�Ч������ҩ������Ҫ�ɷ�Ϊ����ң����ж��ֺϳ�·�ߣ���ͼ����һ�ֺϳɷ�����
��֪±���������·�Ӧ��R��Cl+NaCN![]() R��C��N+NaCl��
R��C��N+NaCl��

�ش��������⣺
(1)д��D�Ľṹ��ʽ��____________________��
(2)д����Ӧ���ͣ�A��B____________��B��C____________��
(3)C�ͱ��ӵĹ�ϵ��____________��
a.��Ϊͬ���칹�� b.��Ϊͬϵ��
c.��Ϊ�����廯���� d.�����ڷ�����
(4)д��C������ȥ��Ӧ�Ļ�ѧ����ʽ(ע����Ӧ����)��__________________________��
(5)��A������ͬ����ʽ���ұ�����ֻ��һ��ȡ�������л��ﹲ�����֣���A�Ľṹ�⣬д���������ֽṹ��ʽ�е��������֣�
_____________________________��____________________________��
2.�����ʽṹ�����ʡ��������ƳɵĽ���ȼ�ϡ��ǽ�������ȼ�ϡ���������Ӧ�õ��������߿Ƽ�����
A��B�ĵ��ʵ�λ������ȼ���ȴ�����ȼ�ϡ���֪A��BΪ������Ԫ�أ���ԭ�ӵĵ�һ�����ĵ��������±���ʾ��
������/kJ��mol-1 | I1 | I2 | I3 | I4 |
A | 932 | 1 821 | 15 390 | 21 771 |
B | 738 | 1 451 | 7 733 | 10 540 |

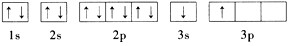
(1)ijͬѧ����������Ϣ���ƶ�B�ĺ�������Ų�����ͼ��ʾ����ͬѧ�����ĵ����Ų�ͼΥ����____________��
(2)���ݼ۲���ӶԻ������ۣ�Ԥ��A����Ԫ���γɵļ��ӿռ乹��Ϊ____________��
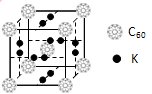
(3)������Ϊһ�������Դ�����������Ĵ������⣬C60������������ϡ�
(4)��֪���ʯ�е�C��C�ļ���Ϊ154.45 pm��C60��C��C����Ϊ145��140 pm����ͬѧ�ݴ���ΪC60���۵���ڽ��ʯ������Ϊ�Ƿ���ȷ����������_________________________��
(5)��ѧ�Ұ�C60��K������һ��������һ�ָ���ϩ������侧����ͼ��ʾ���������ڵ���ʱ��һ�ֳ����塣�����ʵ�Kԭ�Ӻ�C60���ӵĸ�����Ϊ____________��

(6)��C60��ѧ���ֺϳ���Si60��N60��C��Si��Nԭ�ӵ縺���ɴ�С��˳����__________��Si60������ÿ����ԭ��ֻ�����ڵ�3����ԭ���γɹ��ۼ�����ÿ����ԭ������㶼����8�����ȶ��ṹ����Si60�����Цм�����ĿΪ____________��