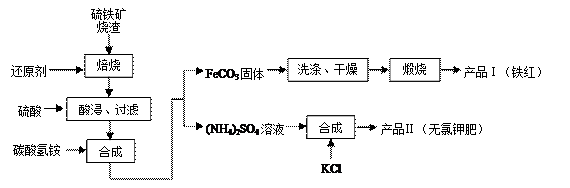
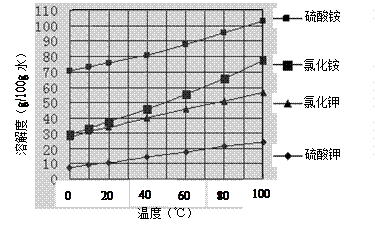
��Ŀ����
��5�֣���ˮ��һ����Ҫ����Ȼ��Դ�����������жԺ�ˮ��Դ�IJ������á�
(1)�Ӻ�ˮ�л���Ȼ��ơ�����ˮ���� �ɵõ����Σ�Ϊ��ȥ�����к��е�SO42-��Ca2+��Mg2+�����ʣ������²��������ܽ⣻�ڼӹ�����Na2CO3��Һ�� �ۼӹ�����BaCl2��Һ���ܼ�����������ݼӹ���NaOH��Һ���������ᾧ���߹��ˡ���ȷ�IJ���˳����_________________��(�������һ�ֺ������)��
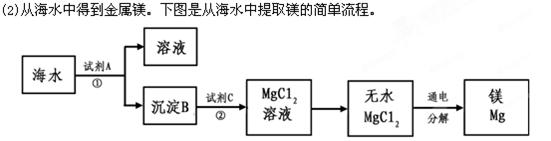 ���������У�����B���Լ�C���������кͷ�Ӧ�������B�Ļ�ѧʽΪ__________��
���������У�����B���Լ�C���������кͷ�Ӧ�������B�Ļ�ѧʽΪ__________��
����ˮMgCl2��ȡMg�Ļ�ѧ����ʽΪ_____________________________________����
ˮ�������Ǻ���MgCl2����Һ������ͨ������١��ڵõ���MgCl2��Һ�кβ�ͬ��___________________________________________________________��
(1)�Ӻ�ˮ�л���Ȼ��ơ�����ˮ���� �ɵõ����Σ�Ϊ��ȥ�����к��е�SO42-��Ca2+��Mg2+�����ʣ������²��������ܽ⣻�ڼӹ�����Na2CO3��Һ�� �ۼӹ�����BaCl2��Һ���ܼ�����������ݼӹ���NaOH��Һ���������ᾧ���߹��ˡ���ȷ�IJ���˳����_________________��(�������һ�ֺ������)��
 ���������У�����B���Լ�C���������кͷ�Ӧ�������B�Ļ�ѧʽΪ__________��
���������У�����B���Լ�C���������кͷ�Ӧ�������B�Ļ�ѧʽΪ__________������ˮMgCl2��ȡMg�Ļ�ѧ����ʽΪ_____________________________________����
ˮ�������Ǻ���MgCl2����Һ������ͨ������١��ڵõ���MgCl2��Һ�кβ�ͬ��___________________________________________________________��
�����ᾧ �٢ۢڢݢߢܢ�(��٢ݢۢڢߢܢ�٢ۢݢڢߢܢ�)
��3�� Mg(OH)2 MgCl2ͨ�� Mg+Cl2�� �����Ǿ��������ͷ�������Һ(��ˮ�к����Ȼ��Ƶȶ������ʣ��Ȼ�þ��Ũ�Ⱥܵ�)
��3�� Mg(OH)2 MgCl2ͨ�� Mg+Cl2�� �����Ǿ��������ͷ�������Һ(��ˮ�к����Ȼ��Ƶȶ������ʣ��Ȼ�þ��Ũ�Ⱥܵ�)
��

��ϰ��ϵ�д�
 ������ĩ��ϰ��ѵ��ϵ�д�
������ĩ��ϰ��ѵ��ϵ�д� С��ʿ��ĩ����100��ϵ�д�
С��ʿ��ĩ����100��ϵ�д�
�����Ŀ
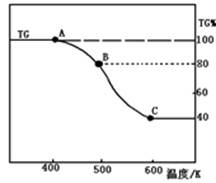
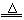 MgO + H2O
MgO + H2O 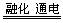 Mg + Cl2��
Mg + Cl2�� �����ͻ�������β�����к������ŷ�
�����ͻ�������β�����к������ŷ�