��Ŀ����
�״�����Ҫ�Ļ�ѧ��ҵ����ԭ�Ϻ�Һ��ȼ�ϡ���ҵ�Ͽ�����CO��CO2������ȼ�ϼ״�����֪�״��Ʊ����йػ�ѧ��Ӧ�Լ��ڲ�ͬ�¶��µĻ�ѧ��Ӧƽ�ⳣ�����±���ʾ��
��ѧ��Ӧ | ƽ�ⳣ�� | �¶�(�棩 | |
500 | 800 | ||
��2H2(g��+CO(g�� | K1 | 2.5 | 0.15 |
��H2(g��+CO2(g�� | K2 | 1.0 | 2.50 |
��3H2(g��+CO2(g�� | K3 | ||
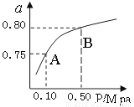
��1��ij�¶��·�Ӧ����H2��ƽ��ת����(a������ϵ��ѹǿ(P���Ĺ�ϵ��ͼ��ʾ����ƽ��״̬��A�䵽Bʱ��ƽ�ⳣ��K(A�� K(B��(���������������������
��2����Ӧ���� (����ȡ����ȡ�����Ӧ��
��3���жϷ�Ӧ�ۡ�H 0����S 0(�>����=����<����
��500�桢2L���ܱ������У����з�Ӧ�ۣ����ijʱ��H2��CO2�� CH3OH��H2O�����ʵ����ֱ�Ϊ6mol��2 mol��10 mol��10 mol����ʱv(���� v(�棩 (�>����=����<����
��4��һ���¶��£���3 L�ݻ��ɱ���ܱ������з�����Ӧ�ڣ���֪c(CO���뷴Ӧʱ��t�仯���ߢ���ͼ��ʾ������t 0ʱ�̷ֱ�ı�һ�����������ߢ��Ϊ���ߢ�����ߢ�
0ʱ�̷ֱ�ı�һ�����������ߢ��Ϊ���ߢ�����ߢ�

�����ߢ��Ϊ���ߢ�ʱ���ı�������� ��
�����ߢ��Ϊ���ߢ�ʱ���ı�������� ��
��5��һ���¶��£�C(s����ˮ�������ܱ������У�����ƽ��״̬��C(s��+H2O(g�� CO(g��+H2(g������H>0��
CO(g��+H2(g������H>0��
��ƽ��ʱ��������ƽ����Է�������Mr(ƽ����ȡֵ��ΧΪ__________>Mr(ƽ��>__________��
����ʹ��CO��H2���淴Ӧ��������ͬ�¶��½���ƽ�⣬��ƽ���������ƽ����Է�������M��r(ƽ����ȡֵ��ΧΪ__________>M��r(ƽ��>__________��
��CH4 ��C2H4 ��C2H6 ��C3H4 ��C3H8 ��C3H6��
| A�� | �ۢ� | B�� | �ڢ� | C�� | �٢ڢ� | D�� | �٢ڢ� |
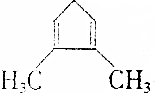 ����������Ȼ�̼��Һ��Ӧ����������У�������
����������Ȼ�̼��Һ��Ӧ����������У�������| A�� | 2�� | B�� | 3�� | C�� | 4�� | D�� | 5�� |
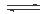 CH3OH(g��
CH3OH(g��

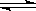 CO(g��+H2(g����һ�ܱ������н��У�����˵��������ܹ���������( ��
CO(g��+H2(g����һ�ܱ������н��У�����˵��������ܹ���������( ��