��Ŀ����
��һ�����Ϊ2L���ܱ������У������·������з�Ӧ��
����Fe(s) �� CO2(g) FeO(s) �� CO(g) + Q kJ
FeO(s) �� CO(g) + Q kJ
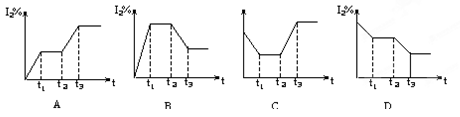
����CO2��CO�����ʵ�����mol����ʱ�䣨min���ı仯��ͼ��ʾ��
(1)����Ӧ��1minʱ��һ�δﵽƽ��״̬�����������������3.2g����CO��Ũ�ȱ仯��ʾ�ķ�Ӧ���ʦ�(CO)=_________��
(2)����Ӧ������2minʱ����ֻ�ı�һ�����������߷����ı仯��ͼ��ʾ��3minʱ�ٴδﵽƽ�⣬��Q 0�����������������=��������һ��ƽ����ڶ���ƽ���ƽ�ⳣ����ȣ�K1 K2��(���������������=��)��
(3)��5minʱ�ٳ���һ������CO(g)��ƽ�ⷢ���ƶ�������˵����ȷ���� ����д��ţ���
a����(��)��������С b����(��)�ȼ�С������
c����(��)��������С d����(��)�ȼ�С������
��ʾn(CO2)�仯��������________����дͼ�����ߵ���ĸ��ţ���
(4)�����ù�̬���ʵ��й���������˵���÷�Ӧ�Ѿ��ﵽ��ѧƽ��״̬��
______________________________________________________________________��
����Fe(s) �� CO2(g)
 FeO(s) �� CO(g) + Q kJ
FeO(s) �� CO(g) + Q kJ����CO2��CO�����ʵ�����mol����ʱ�䣨min���ı仯��ͼ��ʾ��

(1)����Ӧ��1minʱ��һ�δﵽƽ��״̬�����������������3.2g����CO��Ũ�ȱ仯��ʾ�ķ�Ӧ���ʦ�(CO)=_________��
(2)����Ӧ������2minʱ����ֻ�ı�һ�����������߷����ı仯��ͼ��ʾ��3minʱ�ٴδﵽƽ�⣬��Q 0�����������������=��������һ��ƽ����ڶ���ƽ���ƽ�ⳣ����ȣ�K1 K2��(���������������=��)��
(3)��5minʱ�ٳ���һ������CO(g)��ƽ�ⷢ���ƶ�������˵����ȷ���� ����д��ţ���
a����(��)��������С b����(��)�ȼ�С������
c����(��)��������С d����(��)�ȼ�С������
��ʾn(CO2)�仯��������________����дͼ�����ߵ���ĸ��ţ���
(4)�����ù�̬���ʵ��й���������˵���÷�Ӧ�Ѿ��ﵽ��ѧƽ��״̬��
______________________________________________________________________��
0.1mol/(L��min)��2�֣�
��������1��1�𣬹�2�֣�
c �� b ��1��1�𣬹�2�֣�
Fe(��FeO)������(�����ʵ���)���ֲ��䣻�������������ֲ��䡣��2�֣�
(1)�����������������3.2g�����ݻ�ѧ��Ӧ����ʽ����֪��0.2mol FeO���ɣ�����1min��Ҳ������0.2molCO����(CO)= 0.1mol/(L��min)
(2)��Ӧ������2minʱ���ı�����������£���Ϊ��Ӧ���ʼӿ죬ƽ�����ƣ�����Ӧ�����ȷ�Ӧ��Q��0�����£�ƽ�����ƣ�K��������K1 <K2
(3) 5minʱ�ٳ���һ������CO(g)��ƽ�������ƶ�����(��)ͻȻ����Ȼ���С����(��)������ѡC��
��ʾn(CO2)�仯��������b��ƽ�����ƣ�n(CO2)������
(4) Fe(��FeO)������(�����ʵ���)���ֲ��䣻�������������ֲ��䣬����˵�����淴Ӧ�Ѿ��ﵽ��ѧƽ��״̬��
(2)��Ӧ������2minʱ���ı�����������£���Ϊ��Ӧ���ʼӿ죬ƽ�����ƣ�����Ӧ�����ȷ�Ӧ��Q��0�����£�ƽ�����ƣ�K��������K1 <K2
(3) 5minʱ�ٳ���һ������CO(g)��ƽ�������ƶ�����(��)ͻȻ����Ȼ���С����(��)������ѡC��
��ʾn(CO2)�仯��������b��ƽ�����ƣ�n(CO2)������
(4) Fe(��FeO)������(�����ʵ���)���ֲ��䣻�������������ֲ��䣬����˵�����淴Ӧ�Ѿ��ﵽ��ѧƽ��״̬��

��ϰ��ϵ�д�
�����Ŀ
 pC(g)���ܱ������н��У���ͼ��ʾ�ڲ�ͬ��Ӧʱ��t ʱ���¶�T��ѹǿP��������C�ڻ�����е�����ٷֺ����Ĺ�ϵ���ߡ�
pC(g)���ܱ������н��У���ͼ��ʾ�ڲ�ͬ��Ӧʱ��t ʱ���¶�T��ѹǿP��������C�ڻ�����е�����ٷֺ����Ĺ�ϵ���ߡ�
 CO(g)+H2(g) ,��H>0,�ﵽƽ�⣬����˵����ȷ����( )
CO(g)+H2(g) ,��H>0,�ﵽƽ�⣬����˵����ȷ����( ) 2HI(g)�����������˵���÷�Ӧһ���ﵽƽ��״̬����
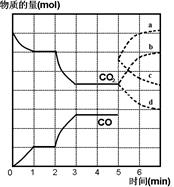
2HI(g)�����������˵���÷�Ӧһ���ﵽƽ��״̬���� N2O4(g) ��H<0���ֽ�һ����NO2��N2O4�Ļ������ͨ�����Ϊ2 L�ĺ����ܱ������У���Ӧ��Ũ����ʱ��仯��ϵ����ͼ��ʾ��
N2O4(g) ��H<0���ֽ�һ����NO2��N2O4�Ļ������ͨ�����Ϊ2 L�ĺ����ܱ������У���Ӧ��Ũ����ʱ��仯��ϵ����ͼ��ʾ��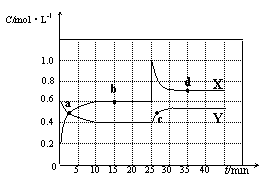
 N2O4(g)��b���ƽ�ⳣ��K(b)= ��
N2O4(g)��b���ƽ�ⳣ��K(b)= ��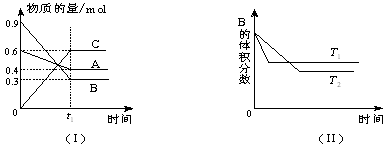
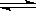 x Q(g) +3R(g)���÷�Ӧ��ƽ��ʱ������2.4mol R�������Q��Ũ��Ϊ0.4 mol/L�������й�������ȷ����
x Q(g) +3R(g)���÷�Ӧ��ƽ��ʱ������2.4mol R�������Q��Ũ��Ϊ0.4 mol/L�������й�������ȷ���� z C
z C
 ��
��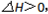 ����t1ʱ��ʱ�ﵽƽ�⣬t2ʱ�������²����֣���t3ʱ��ʱ�ִﵽ�µ�ƽ�⣬������һ�仯��ͼ���ǣ�
����t1ʱ��ʱ�ﵽƽ�⣬t2ʱ�������²����֣���t3ʱ��ʱ�ִﵽ�µ�ƽ�⣬������һ�仯��ͼ���ǣ�