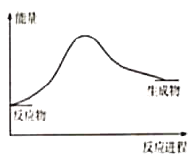
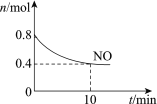
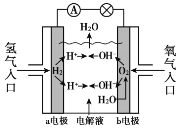
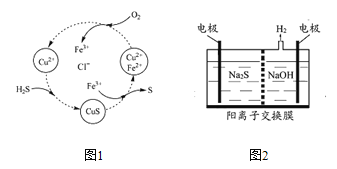
��Ŀ����
����Ŀ�����������Ҵ��Ǻϳ�������Ѫ�ܲ�����Ҫҩ�����������İ����м��塣����һ�ֺϳ�·����ͼ��
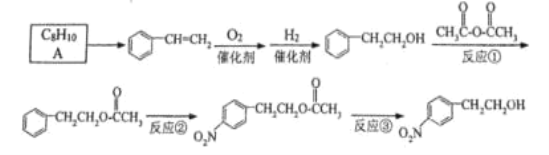
��1��A�Ľṹ��ʽΪ_____��ʵ������A��ȡ����ϩͨ����Ҫ����������Ӧ����һ���ķ�Ӧ�Լ�������ΪCl2/���գ��ڶ����Ļ�ѧ��Ӧ����ʽΪ_____��
��2����Ӧ�ڵķ�Ӧ����Ϊ��Ӧ_____����Ʒ�Ӧ�١���Ӧ�۵�Ŀ����_____��
��3����Ӧ�۵��Լ�������Ϊ_____��
��4���������Ǻϳ����в�������Ʒ�֣��ʺ�����̥��������ȡ���������1��3������ϩ�ͱ���ϩ���۵õ���д�������Ľṹ��ʽ_____��
��5����1��3������ϩΪԭ�Ͽ��Ժϳ��л�����ԭ��1��4����������HOCH2CH2CH2CH2OH��д����ϳ�·��______��
���ϳ�·�߳��õı�ʾ��ʽΪ��A![]() B
B![]() ����Ŀ����
����Ŀ����
���𰸡�
 +NaOH
+NaOH![]()
 +NaCl+H2O����
+NaCl+H2O����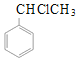 + NaOH
+ NaOH![]()
 +NaCl+H2O�� ȡ��(������)��Ӧ ������������OH����ֹ�䱻���� NaOH��Һ������
+NaCl+H2O�� ȡ��(������)��Ӧ ������������OH����ֹ�䱻���� NaOH��Һ������ 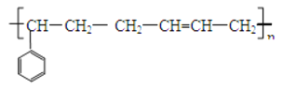 CH2=CH-CH=CH2
CH2=CH-CH=CH2![]() CH2BrCH=CHCH2Br
CH2BrCH=CHCH2Br ![]() HOCH2CH=CHCH2OH
HOCH2CH=CHCH2OH![]() HOCH2CH2CH2CH2OH
HOCH2CH2CH2CH2OH
��������
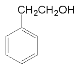
���ݺϳ�·�߷�����֪��A�ȷ���ȡ����Ӧ�õ�±�����������������ƵĴ���Һ�м��ȷ�����ȥ��Ӧ�õ�����ϩ( )����AΪ�ұ�(
)����AΪ�ұ�( )��
)�� ���������ӳɵõ�
���������ӳɵõ�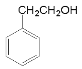 ��
�� ��
��![]() ����ȡ����Ӧ�õ�
����ȡ����Ӧ�õ�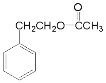 ��
�� ��Ũ������Ũ���ᡢ���ȵ������·�������(��ȡ��)��Ӧ�õ�
��Ũ������Ũ���ᡢ���ȵ������·�������(��ȡ��)��Ӧ�õ�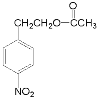 ��
�� ��NaOH��Һ�����ȷ���ˮ�ⷴӦ�õ�
��NaOH��Һ�����ȷ���ˮ�ⷴӦ�õ�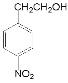 ���ݴ˷���������⡣
���ݴ˷���������⡣
(1)������������֪��AΪ�ұ�����ṹ��ʽΪ ��ʵ������A��ȡ����ϩͨ����Ҫ����������Ӧ����һ���ķ�Ӧ�Լ�������ΪCl2/���յõ�±�������ڶ����ķ�ӦΪ±�������������ƵĴ���Һ�м��ȷ�����ȥ��Ӧ�õ�����ϩ����Ӧ����ʽΪ
��ʵ������A��ȡ����ϩͨ����Ҫ����������Ӧ����һ���ķ�Ӧ�Լ�������ΪCl2/���յõ�±�������ڶ����ķ�ӦΪ±�������������ƵĴ���Һ�м��ȷ�����ȥ��Ӧ�õ�����ϩ����Ӧ����ʽΪ +NaOH
+NaOH![]()
 +NaCl+H2O����
+NaCl+H2O���� + NaOH
+ NaOH![]()
 +NaCl+H2O�����ʴ�Ϊ��
+NaCl+H2O�����ʴ�Ϊ�� ��
�� +NaOH
+NaOH![]()
 +NaCl+H2O����
+NaCl+H2O���� + NaOH
+ NaOH![]()
 +NaCl+H2O����
+NaCl+H2O����
(2) ��Ũ������Ũ���ᡢ���ȵ������·�������(��ȡ��)��Ӧ�õ�
��Ũ������Ũ���ᡢ���ȵ������·�������(��ȡ��)��Ӧ�õ� ��������OH���л�ԭ�ԣ�����Ũ���ᷢ��������ԭ��Ӧ������Ʒ�Ӧ�١���Ӧ�۵�Ŀ���ǣ�������OH����ֹ�䱻�������ʴ�Ϊ��ȡ��(������)��Ӧ��������������OH����ֹ�䱻������
��������OH���л�ԭ�ԣ�����Ũ���ᷢ��������ԭ��Ӧ������Ʒ�Ӧ�١���Ӧ�۵�Ŀ���ǣ�������OH����ֹ�䱻�������ʴ�Ϊ��ȡ��(������)��Ӧ��������������OH����ֹ�䱻������
(3)��Ӧ��Ϊ ��NaOH��Һ������ˮ��õ�
��NaOH��Һ������ˮ��õ� ��CH3COONa���ʴ�Ϊ��NaOH��Һ�����ȣ�
��CH3COONa���ʴ�Ϊ��NaOH��Һ�����ȣ�
(4)���������Ϣ����������1��3������ϩ(CH2=CH-CH=CH2)�ͱ���ϩ���۵õ������Ľṹ��ʽΪ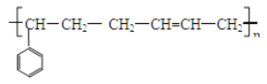 ���ʴ�Ϊ��
���ʴ�Ϊ��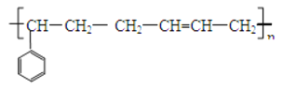 ��
��
(5)��1��3������ϩΪԭ���Ʊ�1��4����������������1��3������ϩ���巢��1��4���ӳɣ�Ȼ��ˮ������ϩ�������������ӳɼ��ɣ��ϳ�·��ΪCH2=CH-CH=CH2![]() CH2BrCH=CHCH2Br
CH2BrCH=CHCH2Br ![]() HOCH2CH=CHCH2OH
HOCH2CH=CHCH2OH![]() HOCH2CH2CH2CH2OH���ʴ�Ϊ��CH2=CH-CH=CH2
HOCH2CH2CH2CH2OH���ʴ�Ϊ��CH2=CH-CH=CH2![]() CH2BrCH=CHCH2Br
CH2BrCH=CHCH2Br ![]() HOCH2CH=CHCH2OH
HOCH2CH=CHCH2OH![]() HOCH2CH2CH2CH2OH��
HOCH2CH2CH2CH2OH��

 ���100��1�ž�ϵ�д�
���100��1�ž�ϵ�д�