ЬтФПФкШн
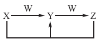
КЯГЩАБЙЄвЕЩњВњжаЫљгУЕФІС?FeДпЛЏМСЕФжївЊГЩЗжЪЧFeOЁЂFe2O3ЁЃ
ЃЈ1ЃЉФГFeOЁЂFe2O3ЛьКЯЮяжаЃЌЬњЁЂбѕЕФЮяжЪЕФСПжЎБШЮЊ4ЁУ5ЃЌЦфжаFe2ЃЋгыFe3ЃЋЮяжЪЕФСПжЎБШЮЊ________ЁЃ
ЃЈ2ЃЉЕБДпЛЏМСжаFe2ЃЋгыFe3ЃЋЕФЮяжЪЕФСПжЎБШЮЊ1ЁУ2ЪБЃЌЦфДпЛЏЛюадзюИпЃЌДЫЪБЬњЕФбѕЛЏЮяЛьКЯЮяжаЬњЕФжЪСПЗжЪ§ЮЊ________ЃЈгУаЁЪ§БэЪОЃЌБЃСє2ЮЛаЁЪ§ЃЉЁЃ
ЃЈ3ЃЉвдFe2O3ЮЊдСЯжЦБИЩЯЪіДпЛЏМСЃЌПЩЯђЦфжаМгШыЪЪСПЬМЗлЃЌЗЂЩњШчЯТЗДгІЃК2Fe2O3ЃЋC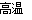 4FeOЃЋCO2ЁќЁЃ
4FeOЃЋCO2ЁќЁЃ
ЃЈ1ЃЉ1ЁУ1ЁЁЃЈ2ЃЉ0.72ЁЁЃЈ3ЃЉ6
ЁОНтЮіЁПЃЈ1ЃЉЩшFe2ЃЋЕФЮяжЪЕФСПЮЊxЃЌИљОнЛЏКЯМлДњЪ§КЭЮЊ0ЕФддђЃК2xЃЋЃЈ4ЃxЃЉЁС3ЃН5ЁС2ЃЌxЃН2 molЃЌЙЪFe3ЃЋЕФЮяжЪЕФСПвВЮЊ2 molЁЃFe2ЃЋгыFe3ЃЋЕФЮяжЪЕФСПжЎБШЮЊ1ЁУ1ЁЃ
ЃЈ2ЃЉЩшДпЛЏМСжаКЌ1 mol Fe2ЃЋЁЂ2 mol Fe3ЃЋЃЌИљОнЛЏКЯМлДњЪ§КЭЮЊ0ЃЌПЩжЊДпЛЏМСжабѕдзгЕФЮяжЪЕФСПЮЊ1 molЃЋ3 molЃН4 molЁЃДпЛЏМСжаЬњЕФжЪСПЗжЪ§ЮЊ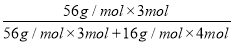 Ёж0.72ЁЃ
Ёж0.72ЁЃ
ЃЈ3ЃЉгЩЬтвтжЊЃЌБЛЛЙдЕФFe2O3гыЮДБЛЛЙдЕФFe2O3ЕФЮяжЪЕФСПжЎБШЮЊ1ЁУ2ЃЌБЛЛЙдЕФFe2O3ЕФЮяжЪЕФСПЮЊ ЃН1 molЃЌдђЃК
ЃН1 molЃЌдђЃК
2Fe2O3ЁЁЃЋЁЁC=4FeOЃЋCO2Ёќ
2 mol 12 g
1 mol mЃЈCЃЉ
mЃЈCЃЉЃН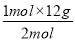 ЃН6 gЁЃ
ЃН6 gЁЃ

 дФЖСПьГЕЯЕСаД№АИ
дФЖСПьГЕЯЕСаД№АИ