��Ŀ����
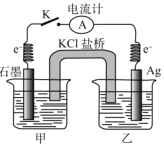
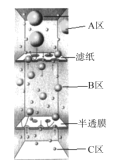
����Ŀ������0.01molFeCl3���Ȼ���������Һ����ñ�û��ǣ������÷�ɢϵ����ͼ��ʾװ�õ� A ������B��������C���Dz��ϸ����е�����ˮ����֪NAΪ�����ӵ�������ֵ������˵������ȷ����
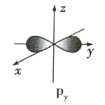
A.ʵ�����Ʊ� Fe(OH)3����ķ�ӦΪ��FeCl3+3H2O![]() Fe(OH)3(����)+3HCl
Fe(OH)3(����)+3HCl
B.��ֽ�ϲ����ĺ��ɫ����ΪFe(OH)3�������
C.��B��������ɫ��ɢϵΪFe(OH)3����
D.����C����H+����ĿΪ0.03NA
���𰸡�D
��������
A������FeCl3�ڷ�ˮ��ˮ������Ʊ����壬��ѧ����ʽΪFeCl3+3H2O![]() Fe(OH)3(����)+3HCl ����ȷ��A��ѡ��
Fe(OH)3(����)+3HCl ����ȷ��A��ѡ��
B����ֽ�ϲ�ķ�ɢϵ����������ֱ��ͨ������10-7 mʱ��Ϊ��Һ����������ֽ�������ֽ�ϵĺ��ɫ����ΪFe(OH)3�����������ȷ��B��ѡ��
C�������ֱ����10-9~10- 7m֮�䣬��������ֽ������������Ĥ���������ֽ�Ͱ�Ĥ֮���B���ɢϵΪ���壬��ȷ��C��ѡ��
D����Fe3����ȫˮ�⣬Cl��ȫ������C�������ݵ���غ㣬�����C����H������ĿӦΪ0.03NA������Fe3����һ����ȫˮ�⣬Cl��Ҳ������ͨ��������ȫ����C��������Fe(OH)3��������ͨ������������ɵ�������H+����������ɣ���˽���C����H������ĿС��0.03NA������Dѡ��
��ѡD��

����Ŀ�������(![]() )���Ʊ��й���֬����Ҫԭ�ϣ�ij�����ֲ�Ʒ�к��б����ἰ�۱���ϩ�����������������
)���Ʊ��й���֬����Ҫԭ�ϣ�ij�����ֲ�Ʒ�к��б����ἰ�۱���ϩ�����������������
���� | ��Է������� | �۵�(��) | �е�(��) | ˮ���ܽ��(25��) |
����ȩ | 106 | -26 | 179.62 | �� |
�۱���ϩ | 104n | 83.1~105 | 240.6 | ���� |
����� | 148 | 135 | 300 | ��(��ˮ������) |
ʵ�����ᴿ�����IJ��輰װ������(����װ��δ����)���Իش�������⣺
2g�ֲ�Ʒ��30mL��ˮ�Ļ����![]()
![]() ��Һ
��Һ![]()
![]() ����
����
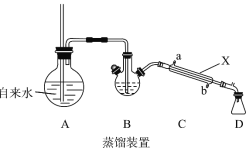
(1)װ��A�г��������ܵ�������_________�������ʹ����ȩ ��ˮ�����뿪ĸҺ������װ����������Ҫ���ȵ�������____________(����ĸ A��B��C��D�ش�)��
(2)����X��������_______����װ������ˮӦ��___________��(��a��b)ͨ�롣
(3)������У�10%NaOH��Һ��������___________���Ա���˳�ȥ�۱���ϩ���ʡ�
(4)������У�֤��ϴ�Ӹɾ�����ѷ�����________������Ʒ�л���������NaCl����һ���ᴿ�������ᾧ�巽��Ϊ_________________��
(5)����ʵ�������ֲ�Ʒ���и�������50%���Ӽ��ܽ�ʱ��ʧ�����10%������ʱ���صõ���Ʒ0.6g�������Ʋ�����ʧ��������ᷴӦ�IJ���ԼΪ_____��(�����ȷ��0.1%)��
����Ŀ��ԭ�������������A��B��C��D��E���ֶ���������Ԫ�صIJ�����Ϣ���±���
Ԫ�ش��� | ��Ϣ |
A | ԭ�ӵ�����������Ϊ�ڲ����������3�� |
B | ��������ǿ�Ķ�����Ԫ�� |
C | ԭ�ӵ������������Ǵ����� |
D | ԭ��������������Aԭ�ӵ�������������һ�� |
E | ����������Ӧ��ˮ��������ǿ������ |
�����������Ϣ�ش����⡣
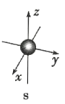
��1��C��������Ļ�ѧʽΪ_______________Aԭ�ӽṹʾ��ͼΪ_______________��
��2��B��C��D�γɵĵ��ʵ��۵�ӵ͵��ߵ�˳��Ϊ_______________����Ԫ�ط��ţ���
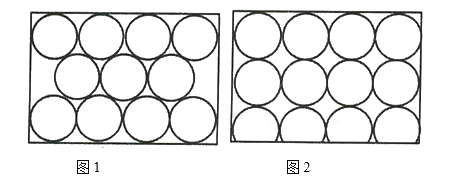
��3��A��C���γɵĻ�����ľ���ṹ���Ȼ��ƾ���ṹ���ƣ�����ÿ����������Χ�Ⱦ��ҽ��ڵ���������ĿΪ_______________�����������������Ӹ�����Ϊ_______________��
��4��A��D���γɾ�����۵��C��E���γɾ�����۵�_______________����������������������ԭ����_______________