��Ŀ����
��14�֣�
�����£���֪HCl��Һ��NaOH��Һ�����ζ���������ͼ��ʾ��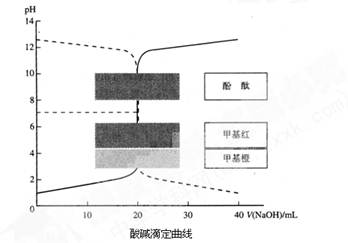
��1����һ������NaOH��Һ�еμ�HCl��Һ������Ϊͼ�� ���ʵ�ߡ������ߡ�����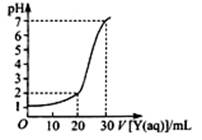
��2����ͼΪ��10mLһ�����ʵ���Ũ�ȵ�NaHSO4��ҺX��һ�����ʵ���Ũ�ȵ�����������ҺY�ζ���ͼ������ͼ���Ƴ�X��Y�����ʵ���Ũ�ȷֱ��� �� ��
��3��ij�о���ѧϰС����о����⣺ʳ������������g/100mL���IJⶨ�����ǽ������µζ�������
A��ȡijƷ�ư״�25.00mL���� �����������ƣ��У�������ˮϡ��10����
B���� �����������ƣ���ȡϡ�ͺ�İ״���Һ20.00mL������250mL��ƿ�У����� ����ָʾ�����ƣ�1��2�Ρ�
C����0.05 mol��L?1NaOH����Һ�ζ������յ㡣���³�ʼ���յ������
��ע�⣺�ζ��ظ�����3�Ρ���
�������ϲ�������������ش��������⡣
�ٲ�����C���У��ζ�ʱ������ע�� ���յ������� ��
�������������в�������ʹ�ⶨ���ƫ�ߵ��� ��
a��ϡ�Ͱ״�����ˮԤ��δ������д���
b��ʢNaOH��Һ�ļ�ʽ�ζ���δ�ñ�Һ��ϴ
c���ζ�ǰ������ȷ���ζ��յ�ʱ���Ӷ���
d���ӽ��յ�ʱ������������ˮϴ����ƿ
�������С���������Ʒ���̱�����ע��һ�£����ܵ�ԭ��֮һ�� ��
��14�֣�
��1������ ��2�֣� ��2��0.09 mol��L?1 0.03 mol��L?1 ��ÿ��2�֣�
��3����A��250mL����ƿ��1�֣�
��B����ʽ�ζ��ܣ�1�֣�����̪��Һ��1�֣�
����ƿ����Һ��ɫ�ı仯����1�֣�
��Һ����ɫ��Ϊdz��ɫ������30s�ڲ���ɫ����1�֣�
��a��b��2�֣�
��ʳ��ϡ��ʱ��������Ʋ�����ʵ��ϡ�ͱ��������������ͱ�����������ȡ���ƫС���ζ��յ���ɫ����30s��ȥ���ζ��������ڼ�ʽ�ζ��ܵļ��촦�����ݣ������̼�ˮ����ϡ�͵ȡ���1�֣�
����

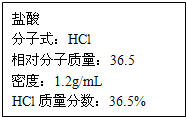 ��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺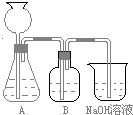
 ͭ����������ʹ�õĽ��������㷺Ӧ���ڵ������Ṥ����е���������ҵ�ȣ���֪Cu2O��H2SO4�ܷ�����Ӧ��Cu2O+H2SO4=Cu+CuSO4+H2O��
ͭ����������ʹ�õĽ��������㷺Ӧ���ڵ������Ṥ����е���������ҵ�ȣ���֪Cu2O��H2SO4�ܷ�����Ӧ��Cu2O+H2SO4=Cu+CuSO4+H2O��