��Ŀ����
�����£������й���Һ��˵������ȷ����
A��ij���ʵ�ˮ��Һ����ˮ�������c(H+)��1��10-a mol/L����a��7�������Һ��pHΪa��14��a
B����0.2 mol/L��ijһԪ����HA��Һ��0.1mol/L NaOH��Һ�������Ϻ���Һ�д��ڣ�2c(OH��)+ c(A��)��2c(H+)��c(HA)
C��pH��3�Ķ�Ԫ����H2R��Һ�� pH��11��NaOH��Һ��Ϻ��Һ��pH����7������Һ��c(R2-) > c(Na+) > c(HR-)
D����ͬ�¶��£�0.2mol/L�Ĵ�����Һ��0.1mol/L�Ĵ�����Һ��c(H+)֮��С��2��1
A��ij���ʵ�ˮ��Һ����ˮ�������c(H+)��1��10-a mol/L����a��7�������Һ��pHΪa��14��a
B����0.2 mol/L��ijһԪ����HA��Һ��0.1mol/L NaOH��Һ�������Ϻ���Һ�д��ڣ�2c(OH��)+ c(A��)��2c(H+)��c(HA)
C��pH��3�Ķ�Ԫ����H2R��Һ�� pH��11��NaOH��Һ��Ϻ��Һ��pH����7������Һ��c(R2-) > c(Na+) > c(HR-)
D����ͬ�¶��£�0.2mol/L�Ĵ�����Һ��0.1mol/L�Ĵ�����Һ��c(H+)֮��С��2��1
C
������A��������Һ������ˮ�ĵ��룮
B��������Һ������ԡ���Һ�ʵ����ԡ������ˮ��Ĺ�ϵ�ȷ�����������Ũ�ȵĹ�ϵ��
C�����ݵ���غ�������غ��ж�����Ũ��֮��Ĺ�ϵ��
D����ͬ�¶��£����������Һ��Ũ��Խ�������̶�ԽС��
�⣺A��ij���ʵ�ˮ��Һ����ˮ�������c��H+��=1��1b-amol/L����a��7��˵������Һ������ˮ�ĵ��룬����������Һ�����Һ�����Ը���Һ��PHֵΪa��14-a����A��ȷ��
B��pH=���6Ԫ����H2R��Һ�� pH=11��NaOH��Һ��Ϻ��Һ��pH����7��˵����Һ�������������������ȣ���Ϊ����������ˮ�����Һ��������Ũ�ȴ���c��R2-����2����ˮ��̶������ģ�����c��R2-����c��HR-������������Ũ�ȴ�С˳����c��Na+����c��R2-����c��HR-������B����
C����b.2 mol/L��ijһԪ����HA��Һ��b.1mol/L NaOH��Һ�������Ϻ���Һ���κ����Ũ�ȶ���b.b��mol/L�����ݵ�������c��OH-��+c��A-��=c��H+��+c��Na+�������������غ��c��A-��+c��HA��=2c��Na+�������Ե�2c��OH-��+c��A-��=2c��H+��+c��HA������C��ȷ��
D����ͬ�¶��֣���b.2mol/L�Ĵ�����Һ��b.1mol/L�Ĵ�����Һ��������ʱ����������Ũ��֮��Ϊ2��1����ʵ����
���������Һ��Ũ��Խ�������̶�ԽС������b.2mol/L�Ĵ�����Һ��b.1mol/L�Ĵ�����Һ��������Ũ��С��2��1����D��ȷ��
��ѡB��

��ϰ��ϵ�д�
 ����ʦ��Сһ����ʦ������ҵϵ�д�
����ʦ��Сһ����ʦ������ҵϵ�д� ���100�ֵ�Ԫ�Ż�������ϵ�д�
���100�ֵ�Ԫ�Ż�������ϵ�д�
�����Ŀ

 �������20.00mL 1.000mol
�������20.00mL 1.000mol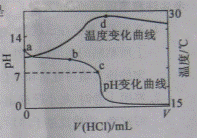



 ��������
�������� ����ǰ��<����
����ǰ��<����