��Ŀ����
��1���ڱ�״���£���ͬ������O2��Cl2��CO2��N2���������������______��
��2��������Ϊm g����������Ͷ����������������ķ��Ӹ�����Ϊ______����ԭ�Ӹ�����Ϊ______����ԭ�Ӹ�����Ϊ______����Ԫ�ص�������Ϊ______��
��3�����ڷ�Ӧ3Cl2+6NaOH=5NaCl+NaClO3+3H2O����������______����ԭ����______����ԭ������______������������______���������뱻��ԭ����ԭ�Ӹ�����Ϊ______������Ӧ������3mol Cl2��ת�Ƶ��ӵ����ʵ���Ϊ______��
�⣺��1����n= =
=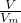 ��֪��V=
��֪��V=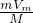 �����������Է�������ԽС����������Խ����Է���������С����N2���������������N2���ʴ�Ϊ��N2��
�����������Է�������ԽС����������Խ����Է���������С����N2���������������N2���ʴ�Ϊ��N2��
��2��n��SO3��=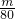 mol��n��SO2��=
mol��n��SO2��=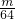 mol����n=
mol����n=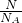 ��֪��
��֪��
�����ķ��Ӹ����ȵ������ʵ���֮�ȣ�N��SO3����n��SO2��=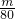 ��
��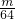 =4��5��
=4��5��
�ɷ�����ɿ�֪��
��ԭ�Ӹ����ȵ��ڷ�����֮��Ϊ4��5��
��ԭ�Ӹ�����Ϊ4��3��5��2=6��5��
��Ԫ�ص������ȵ��ڸ�����Ϊ6��5��
�ʴ�Ϊ��4��5��4��5��6��5��6��5��
��3���ڷ�Ӧ3Cl2+6NaOH=5NaCl+NaClO3+3H2O�У���Ӧǰ��ֻ��ClԪ�صĻ��ϼ۷����仯����Cl2�������������ǻ�ԭ����
���ϼ����ߵIJ���Ϊ����������͵IJ���Ϊ��ԭ�����NaClO3Ϊ�������NaClΪ��ԭ�����Ӧ��ClԪ�ػ��ϼ۷ֱ���0�۱仯Ϊ-1�ۺ�+5�ۣ�
�������뱻��ԭ����ԭ�Ӹ�����Ϊ5��1������Ӧ������3molCl2��ת�Ƶ��ӵ����ʵ���Ϊ5mol��
�ʴ�Ϊ��Cl2��Cl2��NaCl��NaClO3��5��1��5mol��
��������1������n= =
=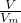 ���㣻
���㣻
��2������n= =
=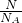 ������ʵķ�����ɼ��㣻
������ʵķ�����ɼ��㣻
��3���ӻ��ϼ۱仯�ĽǶȷ���������ԭ��Ӧ��
���������⿼�����ʵ����ļ����Լ�������ԭ��Ӧ֪ʶ����Ŀ�ѶȲ���ע���й����ʵ����ļ��㣬�ӻ��ϼ۱仯�ĽǶȷ���������ԭ��Ӧ��
 =
=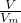 ��֪��V=
��֪��V=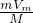 �����������Է�������ԽС����������Խ����Է���������С����N2���������������N2���ʴ�Ϊ��N2��
�����������Է�������ԽС����������Խ����Է���������С����N2���������������N2���ʴ�Ϊ��N2����2��n��SO3��=
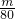 mol��n��SO2��=
mol��n��SO2��=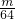 mol����n=
mol����n=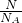 ��֪��
��֪�������ķ��Ӹ����ȵ������ʵ���֮�ȣ�N��SO3����n��SO2��=
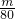 ��
��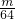 =4��5��
=4��5���ɷ�����ɿ�֪��
��ԭ�Ӹ����ȵ��ڷ�����֮��Ϊ4��5��
��ԭ�Ӹ�����Ϊ4��3��5��2=6��5��
��Ԫ�ص������ȵ��ڸ�����Ϊ6��5��
�ʴ�Ϊ��4��5��4��5��6��5��6��5��
��3���ڷ�Ӧ3Cl2+6NaOH=5NaCl+NaClO3+3H2O�У���Ӧǰ��ֻ��ClԪ�صĻ��ϼ۷����仯����Cl2�������������ǻ�ԭ����
���ϼ����ߵIJ���Ϊ����������͵IJ���Ϊ��ԭ�����NaClO3Ϊ�������NaClΪ��ԭ�����Ӧ��ClԪ�ػ��ϼ۷ֱ���0�۱仯Ϊ-1�ۺ�+5�ۣ�
�������뱻��ԭ����ԭ�Ӹ�����Ϊ5��1������Ӧ������3molCl2��ת�Ƶ��ӵ����ʵ���Ϊ5mol��
�ʴ�Ϊ��Cl2��Cl2��NaCl��NaClO3��5��1��5mol��
��������1������n=
 =
=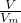 ���㣻
���㣻��2������n=
 =
=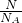 ������ʵķ�����ɼ��㣻
������ʵķ�����ɼ��㣻��3���ӻ��ϼ۱仯�ĽǶȷ���������ԭ��Ӧ��
���������⿼�����ʵ����ļ����Լ�������ԭ��Ӧ֪ʶ����Ŀ�ѶȲ���ע���й����ʵ����ļ��㣬�ӻ��ϼ۱仯�ĽǶȷ���������ԭ��Ӧ��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
���й�������Ħ������ļ���˵����ȷ���ǣ�������
| A��22.4 L�κ���������ʵ�����Ϊ1 mol | B����״���£�1 mol���ʵ����Ϊ22.4 L | C��H2��O2��N2��CO2��ɵĻ������1 mol�ڱ�״���µ����ԼΪ22.4 L | D����ͬ��ͬѹ�£���ͬ������κ����嵥��������������ԭ��������ͬ |
 ��1���ڱ�״���£�����A���ܶ�Ϊ1.25g/L������B������������ܶ�Ϊ21����8.96LA��B�Ļ�����������Ϊ13.44g����������A��B�������Ϊ
��1���ڱ�״���£�����A���ܶ�Ϊ1.25g/L������B������������ܶ�Ϊ21����8.96LA��B�Ļ�����������Ϊ13.44g����������A��B�������Ϊ