��Ŀ����
15�� ��ͼ��ʾ��װ�ã���ͨ��Դ5min�缫3������������0.64g��
��ͼ��ʾ��װ�ã���ͨ��Դ5min�缫3������������0.64g����ش��������⣺
��1��A�Ǹ����������ǵ��أ��缫1��Ӧ��2H++2e-=H2�����缫6��Ӧʽ��Cu-2e-=Cu2+��
��2�����װ�����й��ռ���224mL���壨��״��������Һ���Ϊ100mL��������ǰ��������仯������ͨ��ǰ
CuSO4��Һ�����ʵ���Ũ��Ϊ0.1mol/L��
��3����ʱ������NaCl��������������Һ�����Ҳ��100mL���������Һ��PH��13.3������ǰ������ޱ仯����
��4����װ���в�������Ag��˵��������������˵����ȷ�𣿴���ԭ���DZ�װ����������ͭʧ���ӵõ�ͭ���ӵķ�Ӧ��ͭ���ӽ������ʣ��������ӵõ�����ϣ�ͭ���ӻ����Եõ����ӣ�������Ӧ��
���� ��ͨ��Դ5min�缫3������������0.64g�����Ե缫3��������4��������5��������6��������B��������A�Ǹ�����1��������2�����������ݵ缫��Ӧʽ��ϵ����غ���м��㣮
��1��A�Ǹ�����1��������������ԭ��Ӧ��6��������Cu�ǻ��õ缫���缫����ʧ���ӣ�
��2���缫3������������0.64g���ݴ˼���ͭ�������ʵ���������Ũ�ȣ�
��3�����ݵ���ת�Ƽ��������ɵ��������Ƶ�Ũ�ȣ���������pH��
��4����װ����������ͭʧ���ӵõ�ͭ���ӵķ�Ӧ��ͭ���ӽ������ʣ����ݾ����ӵķŵ�˳��ȷ���缫��Ӧ��
��� �⣺��ͨ��Դ5min�缫3������������0.64g�����Ե缫3��������4��������5��������6��������B��������A�Ǹ�����1��������2��������
��1��A�Ǹ�������װ���ǵ��أ�1���������缫��ӦʽΪ��2H++2e-=H2����6��������Cu�ǻ��õ缫���缫��ӦʽΪ��Cu-2e-=Cu2+��
�ʴ�Ϊ��������⣻2H++2e-=H2����Cu-2e-=Cu2+��
��2���缫3������������0.64g�����Ե缫3�����������ɽ���ͭ��0.01mol������ͭ���ӵ����ʵ�����0.01mol��Ũ���ǣ�$\frac{0.01mol}{0.1L}$=0.1mol/L���ʴ�Ϊ��0.1mol/L��
��3����A����Һ�������100mL��ͨ��ֱ����5minʱ���ɣ�2�������֪���ɽ���ͭ��0.01mol��ת�Ƶ���0.02mol������n��OH-��=0.02mol��������Һ��c��OH-��=$\frac{0.02mol}{0.1L}$=0.2mol/L������pH=14-lg5=13.3���ʴ�Ϊ��13.3��
��4����װ����������ͭʧ���ӵõ�ͭ���ӵķ�Ӧ��ͭ���ӽ������ʣ��������ӵõ�����ϣ�ͭ���ӻ����Եõ����ӣ�������Ӧ�����Ա�װ���в�������Ag����˵�����������ʴ�Ϊ������װ����������ͭʧ���ӵõ�ͭ���ӵķ�Ӧ��ͭ���ӽ������ʣ��������ӵõ�����ϣ�ͭ���ӻ����Եõ����ӣ�������Ӧ��
���� ���⿼��ѧ�����صĹ���ԭ���Լ��缫��Ӧʽ����д�͵����غ�ļ���֪ʶ��ע��֪ʶ�Ĺ��ɺ������ǹؼ����Ѷ��еȣ�

 ��У����ϵ�д�
��У����ϵ�д���1�����Ҫ����ķ���֤��HAΪ���ᣬֻ����������Һ�ͱ�Ҫ�Ļ�ѧ������ʵ����Ʒ�������ȷ�IJ������ڱ�������Ƭ���Ϸ�һСƬ��ֽ���ò�����պȡ��������Һ���ڸ����pH��ֽ�ϣ������ɫ���Ա���ȷ��pH��
��2��ˮ�ĵ���̶ȣ��ܣ��ޣ��������=��
��3�����HAΪ���ᣬ������м�������PH=2���������Һ����������Ũ���ɴ�С��˳��Ϊc��H+����c��Cl-��=c��A-����c��OH-����
��4�����HAΪǿ�ᣬMOHΪǿ��Ѣٺܰ͢������1��4��ϣ����Ϻ���Һ��PH=11.8��
II�������£���ijһԪ��HA��NaOH��Һ�������ϣ�������Һ��Ũ�Ⱥͻ�Ϻ�������Һ��pH���±���
| ʵ���� | HA���ʵ��� Ũ�ȣ�mol/L�� | NaOH���ʵ� ��Ũ�ȣ�mol/L�� | �����Һ��pH |
| �� | 0.2 | 0.2 | pH=a |
| �� | c1 | 0.2 | pH=7 |
| �� | 0.2 | 0.1 | pH��7 |
| �� | 0.1 | 0.1 | pH=9 |
��2���������������ʵ���������Ӷ������������д��HA���뷽��ʽH?H++A-��
��3�����ҡ��������������c1��0.2 ���������������=������
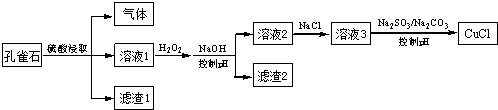
����˵������ȷ���ǣ�������
| A�� | H2O2����Һ1��Fe2+����ΪFe3+����ͨ������pHת��ΪFe��OH��3��ȥ | |
| B�� | SO32-����Һ3�е�Cu2+��ԭ����Ӧ�õ�CuCl | |
| C�� | CO32-�����ǿ�����ҺpH����ʹCuCl���������� | |
| D�� | ���ı��Լ�����˳����Һ3�������뵽������SO32-/CO32-����Һ�У�ͬ������ȡCuCl |
| A�� | NH4Cl | B�� | NH3•H2O | C�� | Cu | D�� | CH3CH2OH |
| A�� | NaCl | B�� | AlCl3 | C�� | CaCl2 | D�� | FeCl3 |
| ���������� | ���� | |
| A | ȡij��Һ�����������ữ��Ba��NO3��2��Һ��������ɫ���� | ����Һһ����SO42- |
| B | NaHCO3��Һ��NaAlO2��Һ��ϲ�����ɫ���� | ���H+��������CO32-��AlO2- |
| C | ij��Һ�����������ʹʯ��ˮ����ǵ���ɫ��ζ���� | ����Һ�϶���HCO3-��CO32-�е�һ�ֻ���� |
| D | �ⶨ��Ũ�ȵ�Na2CO3��Na2SO3��Һ��pH��ǰ��pH�Ⱥ��ߵĴ� | �ǽ����ԣ�S��C |
| A�� | A | B�� | B | C�� | C | D�� | D |

