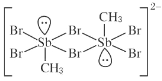
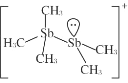
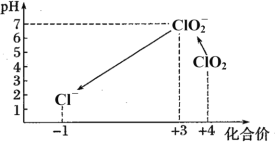
题目内容
【题目】某化学研究性学习小组对某无色水样的成分进行检验,已知该水样中只可能含有K+、Mg2+、Fe3+、Cu2+、Al3+、Ag+、Ca2+、CO32-、SO42-、Cl-中的若干种离子。该小组同学取100 mL水样进行实验,向水样中先滴加硝酸钡溶液,再滴加1 mol·L-1的硝酸,实验过程中沉淀质量的变化情况如图所示:
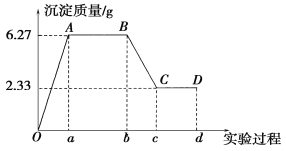
注明:Ob段表示滴加硝酸钡溶液;bd段表示滴加稀硝酸
(1)水样中一定含有的阴离子是________,其物质的量浓度之比为________。
(2)写出BC段所表示反应的离子方程式:__________________________________________。
(3)由B点到C点变化过程中消耗硝酸的体积为________。
(4)试根据实验结果推断K+是否存在?________(填“是”或“否”);若存在,K+的物质的量浓度c(K+)的范围是__________________。(若K+不存在,则不必回答该问)
(5)设计简单实验验证原水样中可能存在的离子:_____________________。(写出实验步骤、现象和结论)
【答案】(1)S![]() 、C
、C![]() 1∶2
1∶2
(2)BaCO3+2H+====Ba2++CO2↑+H2O (3)40 mL
(4)是 c(K+)≥0.6 mol·L-1
(5)取少量水样于试管中,向其中加入过量的硝酸钡溶液和稀硝酸,待沉淀完全后,向上层清液中滴加硝酸银溶液,若产生白色沉淀,则原水样中含Cl-,若不产生白色沉淀,则原水样中不含Cl-
【解析】
试题(1)该水样为无色溶液,Fe3+、Cu2+不存在,依据图像分析加入硝酸钡溶液生成沉淀,加入稀硝酸,沉淀部分溶解证明水样中一定含有S![]() 、C
、C![]() ,又因为C
,又因为C![]() 与Ag+、Ca2+、Mg2+发生反应生成沉淀不能大量存在,所以Ag+、Ca2+、Mg2+不存在;n(S
与Ag+、Ca2+、Mg2+发生反应生成沉淀不能大量存在,所以Ag+、Ca2+、Mg2+不存在;n(S![]() )=
)=![]() =0.01 mol,m(BaCO3)=6.27 g-2.33 g=3.94 g,n(C
=0.01 mol,m(BaCO3)=6.27 g-2.33 g=3.94 g,n(C![]() )=
)=
n(BaCO3)=![]() =0.02 mol;c(S
=0.02 mol;c(S![]() )∶c(C
)∶c(C![]() )=1∶2;(2)BC段所表示反应是碳酸钡溶于稀硝酸的反应,反应的离子方程式为BaCO3+2H+====Ba2++CO2↑+H2O;
)=1∶2;(2)BC段所表示反应是碳酸钡溶于稀硝酸的反应,反应的离子方程式为BaCO3+2H+====Ba2++CO2↑+H2O;
(3)由B点到C点变化过程中依据图像分析溶解的碳酸钡的物质的量n(BaCO3)
=0.02 mol;消耗稀硝酸物质的量为0.04 mol,消耗硝酸的体积=![]() =0.04 L
=0.04 L
="40" mL;(4)依据电解质溶液呈电中性,阳离子K+一定存在;根据电荷守恒得到:
0.01 mol×2+0.02 mol×2+n(Cl-)=n(K+)推知n(K+)≥0.06 mol,则c(K+)≥
0.6 mol·L-1;(5)可能存在的离子是Cl-,实验设计为:取少量水样于试管中,向试管中加入过量的硝酸钡溶液和稀硝酸,待沉淀完全和无气体生成后,向上层清液中滴加适量的硝酸银溶液,若生成白色沉淀,则原水样中含有Cl-,若无白色沉淀生成,证明无Cl-存在。

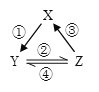
【题目】X、Y、Z 是中学化学中常见的三种物质,下表各组物质之间通过一步反应不能实现图所示转化关系的是
X | Y | Z | 箭头上所标数字的反应条件 | ||
A. | NO | NO2 | HNO3 | ① 常温遇氧气 |
|
B. | Cl2 | NaClO | HClO | ② 通入CO2 | |
C. | Fe | FeCl2 | FeCl3 | ③ 加入Cu | |
D. | Al2O3 | NaAlO2 | Al(OH)3 | ④ 加NaOH溶液 |
A. AB. BC. CD. D