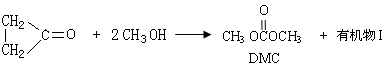
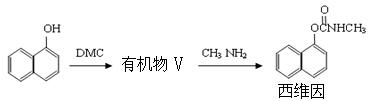��Ŀ����
���ʴ��ֳ��Ҷ����ѣ�����ʽΪC4H10O3��HO-CH2-CH2-O-CH2-CH2-OH�������ʴ���һ����Ҫ�Ļ���ԭ�ϣ�������ȡ�ᡢ�������ȣ���;ʮ�ֹ㷺�����ʴ�һ��ĺϳ�·�����£�

���̢� Br2 ������ ��Ӧ��
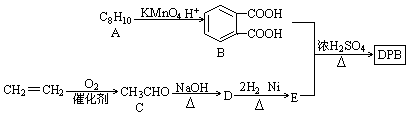
��1�����̢���ʯ�ͼӹ��г��ò��裬������Ϊ ��������B������C�ķ�Ӧ�������� ���÷�Ӧ���� ����д��Ӧ���ͣ�������B������C�Ĺ�������������Ʋ��û���������E��E�����ڽ������ид��B��������E�Ļ�ѧ����ʽ ��
��2��д�������ϳ�·���е�����A��B��C�Ľṹ��ʽ��
A ��B ��C
��3����Ӧ��Ļ�ѧ����ʽΪ�� ��

���̢� Br2 ������ ��Ӧ��
��1�����̢���ʯ�ͼӹ��г��ò��裬������Ϊ ��������B������C�ķ�Ӧ�������� ���÷�Ӧ���� ����д��Ӧ���ͣ�������B������C�Ĺ�������������Ʋ��û���������E��E�����ڽ������ид��B��������E�Ļ�ѧ����ʽ ��
��2��д�������ϳ�·���е�����A��B��C�Ľṹ��ʽ��
A ��B ��C
��3����Ӧ��Ļ�ѧ����ʽΪ�� ��
��1���ѽ⣨1�֣���NaOHˮ��Һ��1�֣���ȡ����ˮ�⣩��Ӧ��1�֣���
CH2BrCH2Br + 2NaOH
 HC��CH��+ 2 NaBr ��1�֣�
HC��CH��+ 2 NaBr ��1�֣���2��A��CH2=CH2��B��Br-CH2-CH2-Br��C��HO-CH2-CH2-OH��1��1��3�֣�
|
 2HO-CH2-CH2-O-CH2-CH2-OH+H2O��1�֣�
2HO-CH2-CH2-O-CH2-CH2-OH+H2O��1�֣������������1��ʯ�͡�����A �� ����B ����ˮ��Ӧ��˵����˫��������Ҫ�ƶ��ʴ�����̼�����٣�ʯ�͡�����A��������̷������ѽⷴӦ���ѽ�������ѻ�������С����ϩ����B��C��BΪ�������C
Ϊ���� ��NaOHˮ��Һ�з�����ˮ�ⷴӦ����ȡ����Ӧ�������и�����Ȳ�������ʡ�
CH2BrCH2Br + 2NaOH
 HC��CH��+ 2 NaBr
HC��CH��+ 2 NaBr ��2���ɺϳ�·�����ƶ��ʴ�:2HO-CH2-CH2-O-CH2-CH2-OH��HO-CH2-CH2-OH��Br-CH2-CH2-Br��CH2=CH2
���ԣ�A��CH2=CH2��B��Br-CH2-CH2-Br��C��HO-CH2-CH2-OH

��ϰ��ϵ�д�
 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д�
�����Ŀ
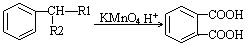
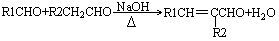
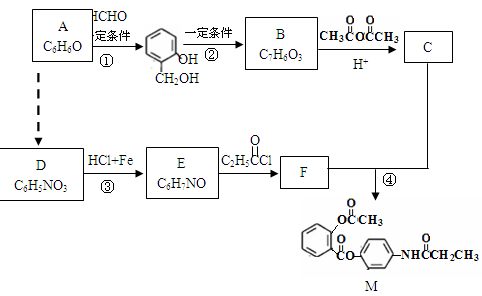 ��֪��
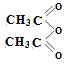
��֪�� 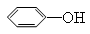 +
+
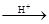
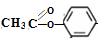 +CH3COOH
+CH3COOH
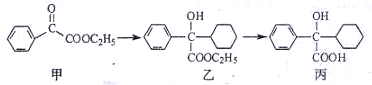
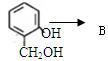 ����һ����ɣ�����Ϳ��ܵ�ԭ��
����һ����ɣ�����Ϳ��ܵ�ԭ��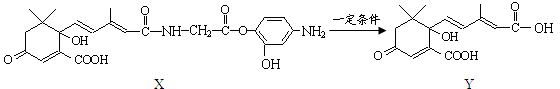

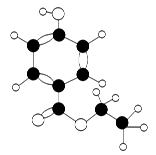
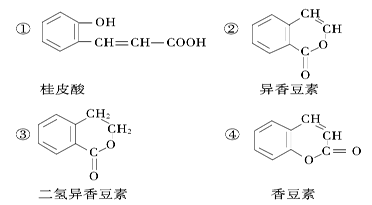
 ������H2CO3Ҳ����д��
������H2CO3Ҳ����д��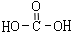 �ṹ������������DMC�ĺϳɷ����ܶ࣬������������
�ṹ������������DMC�ĺϳɷ����ܶ࣬������������