��Ŀ����
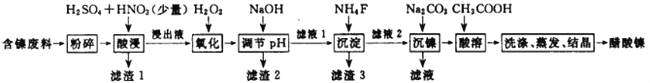
����Ŀ��������[ (CH3COO)2Ni]��һ����Ҫ�Ļ���ԭ�ϡ�һ���Ժ�������(��NiS��Al2O3��FeO��CaO��SiO2) Ϊԭ�ϣ���ȡ�������Ĺ�������ͼ����:
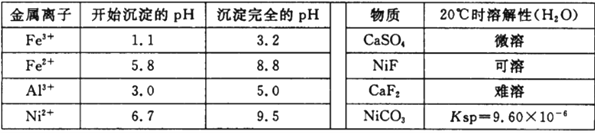
��������������������pH��������ʵ��ܽ������±���
��1��������ĺ����������ʱҪ���Ͻ��裬����ͽ����Ŀ����______________________________��
��2������pH�����У���ҺpH�ĵ��ڷ�Χ��_______________________________��
��3������1������3��Ҫ�ɷֵĻ�ѧʽ�ֱ���____________��_____________��
��4�����������м���H2O2������Ӧ�����ӷ���ʽ________________________________��
��5����������У�lmol NiSʧȥ6NA�����ӣ�ͬʱ����������ɫ�ж����塣д���÷�Ӧ�Ļ�ѧ����ʽΪ____________________________________��
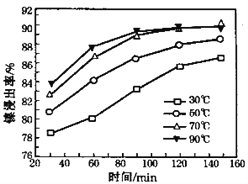
��6�����������������䣬�ڲ�ͬ�¶��¶Ժ��� ���Ͻ������������������ʱ��仯����ͼ�� ���������¶���ʱ��ֱ�Ϊ________�桢 _______min��
���𰸡� ����Ӵ��������߽������ʺͲ��ʣ�������ʣ� 5.0��pH��6.7 SiO2��CaSO4 CaF2 2Fe2++H2O2+2H+= 2Fe3++2H2O NiS+H2SO4+2HNO3== NiSO4+SO2��+2NO��+2H2O 70 120
����������������(�� NiS��Al2O3��FeO��CaO��SiO2)���飬�������������������ˣ�����1Ϊ�������������ƣ�����Һ����Ni2+��Fe2+��Al3+��Ca2+����H2O2��������������ΪFe3+��Ȼ���NaOH����pH��ʹAl3+��Fe3+ת��Ϊ������ͬʱNi2+����ת��Ϊ���������Ե���pH�ķ�Χ5.0��pH��6.7�����ˣ�����2Ϊ����������������������Һ�к���Ni2+��Ca2+���ټӷ���泥�����CaF2���������ˣ�����3ΪCaF2����Һ�м�̼��������NiCO3���������ˣ������мӴ����ܽ⣬����(CH3COO)2Ni��Һ��Ȼ������Ũ������ȴ�ᾧ�õ�(CH3COO)2Ni���塣
(1)������ĺ����������ʱҪ���Ͻ��裬����ͽ����������Ӵ��������߽������ʺͲ��ʣ��ʴ�Ϊ������Ӵ��������߽������ʺͲ��ʣ�
(2)����pHʹAl3+��Fe3+ת��Ϊ������ͬʱNi2+����ת��Ϊ���������ݱ��е����ݿ�֪������pH�ķ�ΧΪ5.0��pH��6.7���ʴ�Ϊ��5.0��pH��6.7��
(3)�����̷�����֪������1Ϊ�������������ƣ�����3ΪCaF2���ʴ�Ϊ��SiO2��CaSO4��CaF2��
(4)��Һ�е��������Ӳ�����ת��Ϊ��������H2O2��������������ΪFe3+����Ӧ�����ӷ���ʽΪ��2Fe2++H2O2+2H+�T2Fe3++2H2O���ʴ�Ϊ��2Fe2++H2O2+2H+�T2Fe3++2H2O��
(5)��������У�1mol NiS����������ʧȥ6NA�����ӣ�ͬʱ����������ɫ�ж����壬������NO��SO2���䷴Ӧ�Ļ�ѧ����ʽΪ��NiS+H2SO4+2HNO3�TNiSO4+SO2��+2NO��+2H2O���ʴ�Ϊ��NiS+H2SO4+2HNO3�TNiSO4+SO2��+2NO��+2H2O��
(6)��ͼ���֪��70����120minʱ�����������ߣ��ʴ�Ϊ��70��120��