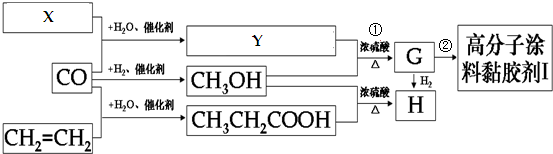
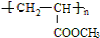��Ŀ����
6����������Ҫ�Ļ�����Ʒ����l��ij������Na2S Ϊԭ�����ù�ҵ��Һ���� H+��Zn2+��Cl2+��Al3+��Fe2+��Fe3+ �����Ƶ�ZnS�����������£�
�ٲ��裨i�����������к�����Ԫ�ص�����ΪAl��OH��3���ѧʽ����
�ڲ��裨i������ZnO������Ϊ����pH����ȥ�����ӣ�
�۲��裨iii���еõ�Cd���ʵ����ӷ���ʽΪZn+Cd2+=Cd+Zn2+��
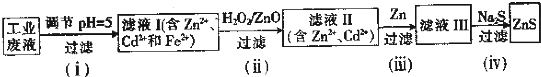
��2����ˮ�й��������Ƴ�ȥ�����ij�ȥ����ҺpH������ʵ������������������ֵ�Ĺ�ϵ��ͼ1��ʾ��Ϊʹ�����ﵽ���Ч����Ӧ����������x=12��pH����9��10֮�䣮
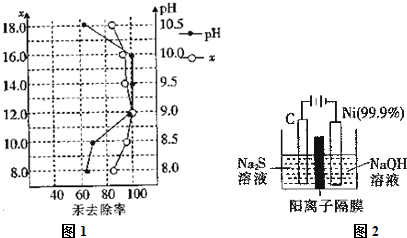
��3�����������Һ��������;�㷺�Ķ������ͼ2�ǵ������������ʵ��װ�ã�
����֪�����ķ�ӦΪxS2--2xe-�TSx���������ĵ缫��Ӧʽ��2H++2e-=H2��������Ӧת��1mol����ʱ���������������Ϊ11.2L ����״���£���
�ڽ�Na2S.9H2O����ˮ������������Һʱ��ͨ�����ڵ����������ܽ⣮��ԭ����2S2-+O2+2H2O=2S��+4OH-�������ӷ�Ӧ����ʽ��ʾ����
���� ��1����Na2S Ϊԭ�����ù�ҵ��Һ���� H+��Zn2+��Cl2+��Al3+��Fe2+��Fe3+ ���Ƶ�ZnS�������̣��ȳ��������ӣ��ټ���˫��ˮ����������������Ϊ�����ӣ���������п����pH����ȥ�����ӣ�����Һ�м������п�û������������ݴ˻ش��ж��Լ������ü��ɣ�
��2������ͼ�������߷������ó���ȥ������ѵ�PH��x��ȡֵ��
��3���ٸ��ݵ��صĹ���ԭ���������Ϸ����õ��ӵĻ�ԭ��Ӧ�������Ϸ���ʧȥ���ӵ�������Ӧ���ش�
�������Ӿ���ǿ�Ļ�ԭ�ԣ��ױ���������Ϊ���ʣ��ݴ����ش�
��� �⣺��1����Na2S Ϊԭ�����ù�ҵ��Һ���� H+��Zn2+��Cl2+��Al3+��Fe2+��Fe3+ ���Ƶ�ZnS�������̣��ȳ��������ӣ��ټ���˫��ˮ����������������Ϊ�����ӣ���������п����pH����ȥ�����ӣ�����Һ�м������п�û�����������
���ȳ��������ӣ������Լ������ˣ����Եõ�������Ԫ�ص������������������������ʴ�Ϊ��Al��OH��3��
�ڲ��裨i������ZnO������Ϊ����pH�����������γ�����������������ȥ�����ӣ��ʴ�Ϊ������pH����ȥ�����ӣ�
�۲��裨iii��������Һ�м������п�û������������õ�Cd���ʵ����ӷ���ʽΪZn+Cd2+=Cd+Zn2+���ʴ�Ϊ��Zn+Cd2+=Cd+Zn2+��
��2������ͼ�������߷�������ȥ�������PH��x��ȡֵ��x=12 pH����9��10֮��ʱ����ȥ������ӽ�100%���ʴ�Ϊ��x=12��pH����9��10֮�䣻
��3�����������������������ӷ����õ��ӵĻ�ԭ��Ӧ����2H++2e-=H2��������Ӧת��1mol����ʱ������������0.5mol�����Ϊ0.5mol��22.4L/mol=11.2L��
�ʴ�Ϊ��2H++2e-=H2����11.2L��
�������е������Ӿ���ǿ�Ļ�ԭ�ԣ��ױ���������Ϊ���ʣ�����ʱҪ�������������õ�������������2S2-+O2+2H2O=2S��+4OH-���ʴ�Ϊ��2S2-+O2+2H2O=2S��+4OH-��
���� �����������Ʊ��Ŀ����⣬��Ҫ�ǻ�ѧ����ʽ��д�������缫��Ӧʽ����д��֪ʶ�����ջ����ǹؼ�����Ŀ�Ѷ��еȣ�

 ��У����ϵ�д�
��У����ϵ�д�| A�� | ��Ӧ��Ĺ��������л�����̼ | |
| B�� | ��Ӧ��Ĺ�������������Ϊ14.4 g | |
| C�� | ��Ӧ��Ĺ��������е���Cu������Ϊ12.8 g | |
| D�� | ��Ӧ��Ĺ�������������������ʵ���Ϊ0.05 mol |
 A��B��C��D��E���dz���������Ԫ�أ��������ڱ��е�λ����ͼ��
A��B��C��D��E���dz���������Ԫ�أ��������ڱ��е�λ����ͼ�� | A | |||||
| B | C | D | |||
| E | |||||
��2��A��C���γɶ���10�����������з���ΪNH3������ΪNH4+��
��3��C��D��E�ļ����ӵİ뾶��С˳��ΪN3-��O2-��Na+��
��4��A��C���γ����ֳ����Ļ����������P����Է�������Ϊ17���ͻ�����Q����Է�������Ϊ32����
��д��Q�Ľṹʽ��
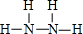
��P��ˮ��Һ������ʴ��H2O2�����������䷴Ӧ�ﲻ��Ⱦ�������仯ѧ����ʽΪ2NH3��H2O+3H2O2=N2��+8H2O��
��5����A��B��C��D��E����Ԫ���еĶ��ֻ��������Ԫ����ɵĻ�����X��Y��Z��W������ת����ϵ��
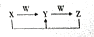 ������Y��Z��ˮ��Һ�д��ڵ���������ͬ��
������Y��Z��ˮ��Һ�д��ڵ���������ͬ����X�������к��еĻ�ѧ�����������Ӽ������ۼ���
�ڷ�ӦX+Z��Y�����ӷ���ʽΪHCO3-+OH-=CO32-+H2O��
��Y��ˮ��Һ������Ũ�ȵĴ�С˳����c��Na+����c��CO32-����c��OH-����c��HCO3-����c��H+����
| A�� | ����Ͷ�ϩ | B�� | ����ͱ��� | C�� | ��ϩ��һ�ȶ��� | D�� | ����ͼ������� |
| A�� | ���Ȼ�������Һ�м����Ȼ�����Һ���Ȼ�����Ksp��С | |
| B�� | ��2.0��10-4 mol/L��K2CrO4��Һ�м���������2.0��10-4mol/AgNO3��Һ������Ag2CrO4�������� | |
| C�� | ��һ������AgCl��Ag2CrO4����������������ˮ�У�����ܽ���ã��ϲ���Һ�е�Cl�����ʵ���Ũ����� | |
| D�� | ��0.001mol/L��AgNO3��Һ��ε���0.001mol/L��KCl��0.001mol/L��K2CrO4�Ļ����Һ�У����Ȳ���AgCl������ |
 ���û�����ϸ�����л��ʵĴ�������������ѧ�ҿ�����������ȼ�ϵ�أ���װ����ͼ��a��bΪ���Ե缫�����ø�װ�ý���ˮ�е��л����C6H12O6Ϊ��������������ȥ���Ӷ��ﵽ����ˮ��Ŀ�ģ�����˵������ȷ���ǣ�������
���û�����ϸ�����л��ʵĴ�������������ѧ�ҿ�����������ȼ�ϵ�أ���װ����ͼ��a��bΪ���Ե缫�����ø�װ�ý���ˮ�е��л����C6H12O6Ϊ��������������ȥ���Ӷ��ﵽ����ˮ��Ŀ�ģ�����˵������ȷ���ǣ�������| A�� | aΪ�������缫��ӦʽΪ��C6H12O6+6H2O-24e-�T6CO2+24H+ | |
| B�� | ��Ӧ�����в����������������ӽ���Ĥ��ɢ�������� | |
| C�� | װ���е����ӽ���Ĥ�������ӽ���Ĥ | |
| D�� | ��װ�ðѵ���ת��Ϊ�������� |