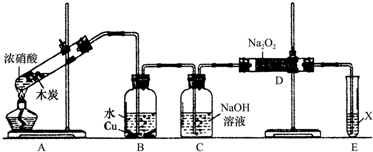
��Ŀ����
4�������仯�������ճ��������й㷺Ӧ�ã���1��д��Fe��OH��2ת��ΪFe��OH��3�Ļ�ѧ����ʽ4Fe��OH��2+O2+2H2O=4Fe��OH��3��
��2���̷���FeSO4•7H2O���Dz�Ѫ����ԭ�ϣ��ױ��ʣ������̷��Ƿ���ʵ��Լ���KSCN��Һ���ʵ������̷��Ƿ���ȫ����ȡ��Ʒ����ˮ���μ����Ը��������Һ������Һ��ɫ�����ʾ��Ʒû����ȫ���ʣ���μ����軯����Һ��������ɫ����������Ʒû����ȫ���ʣ�
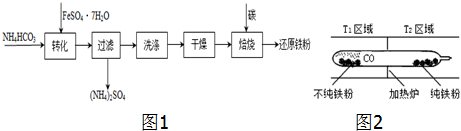
��3�������̷��Ʊ���ԭ���۵Ĺ�ҵ������ͼ1��
�ٸ��������Ҫ��Ϊ����ȥ����ˮ�ͽᾧˮ�������л�������FeCO3•nH2O�ڿ����б�����ΪFeOOH���÷�Ӧ�Ļ�ѧ����ʽΪ4FeCO3•nH2O+O2=4FeOOH+4CO2+��4n-2��H2O
��ȡ������FeCO3��Ʒ12.49g�����գ����յõ���ԭ����6.16g��������Ʒ������FeOOH������0.89g
��4�����ʻ�������Ϊ�������û�ѧƽ���ƶ�ԭ�����롢�ᴿij�����������ۣ�����һЩ����Ӧ�����ʣ�����Ӧװ����ͼ2��Fe��s��+5CO��g��?Fe��CO��5��g����H��0
T1��T2�����������������=�������ж����������ۺ�һ����̼���ϳ��ʻ�����ʱ�ų����������������ںϳ��ӷ����ʻ��������ʻ������ӷ������ʲ����ڲ�������ˣ����ʻ������ӷ����ϸ��¶�����T2ʱ���ʻ������ֽ⣬�����۲������Ҷˣ�һ����̼ѭ�����ã�
���� ��1��Fe��OH��2������ˮ��Ӧ����Fe��OH��3��
��2���̷���FeSO4•7H2O���ױ��ʣ�Fe2+������ΪFe3+�������̷��Ƿ���ʵ��Լ���KSCN��Һ�������̷��Ƿ���ȫ���ʼ�������Ʒ���Ƿ����Fe2+��������Fe2+�Ļ�ԭ�Խ��м���������軯����Һ���飬ʵ�鷽��Ϊȡ��Ʒ����ˮ���μ����Ը��������Һ������Һ��ɫ�����ʾ��Ʒû����ȫ���ʣ�
��3����FeCO3•nH2O����������ΪFeOOH������������ԭ��Ӧ�����غ��ԭ���غ���ƽ��д��
����������Ԫ���غ��������ϵ���㣻
��4�����ۺ�һ����̼���ϳ��ʻ�����ʱ�ų����������������ںϳ��ӷ����ʻ��������ʻ������ӷ������ʲ����ڲ�������ˣ����ʻ������ӷ����ϸ��¶�����T2ʱ���ʻ������ֽ⣬�����۲������Ҷˣ�һ����̼ѭ�����ã�
��� �⣺��1��Fe��OH��2������ˮ��Ӧ����Fe��OH��3����ѧ����ʽ��4Fe��OH��2+O2+2H2O=4Fe��OH��3���ʴ�Ϊ��4Fe��OH��2+O2+2H2O=4Fe��OH��3��
��2���̷���FeSO4•7H2O���ױ��ʣ�Fe2+������ΪFe3+�������̷��Ƿ���ʵ��Լ���KSCN��Һ�������̷��Ƿ���ȫ���ʼ�������Ʒ���Ƿ����Fe2+��������Fe2+�Ļ�ԭ�Խ��м���������軯����Һ���飬ʵ�鷽��Ϊȡ��Ʒ����ˮ���μ����Ը��������Һ������Һ��ɫ�����ʾ��Ʒû����ȫ���ʣ���μ����軯����Һ��������ɫ����������Ʒû����ȫ���ʣ���
�ʴ�Ϊ��KSCN��Һ��ȡ��Ʒ����ˮ���μ����Ը��������Һ������Һ��ɫ�����ʾ��Ʒû����ȫ���ʣ���μ����軯����Һ��������ɫ����������Ʒû����ȫ���ʣ���
��3���ٸ��������Ҫ��Ϊ����ȥ����ˮ�ͽᾧˮ�������л�������FeCO3•nH2O����������ΪFeOOH������������ԭ��Ӧ�ĵ����غ��ԭ���غ㣬д����ѧ����ʽΪ��4FeCO3•nH2O+O2=4FeOOH+4CO2��+��4n-2��H2O���ʴ�Ϊ��4FeCO3•nH2O+O2=4FeOOH+4CO2��+��4n-2��H2O��
������Ʒ��FeCO3�����ʵ���Ϊxmol��FeOOH�����ʵ���Ϊymol����
116x+89y=12.49
x+y=$\frac{6.16}{56}$=0.11
���y=0.01mol����Ʒ������FeOOH������Ϊ0.89g���ʴ�Ϊ��0.89g��
��4����ӦFe��s��+5CO��g��?Fe��CO��5��g����H��0�����ۺ�һ����̼���ϳ��ʻ�����ʱ�ų����������������ںϳ��ӷ����ʻ��������ʻ������ӷ������ʲ����ڲ�������ˣ����ʻ������ӷ����ϸ��¶�����T2ʱ���ʻ������ֽ⣬�����۲������Ҷˣ�һ����̼ѭ�����ã���T1��T2��
�ʴ�Ϊ���������ۺ�һ����̼���ϳ��ʻ�����ʱ�ų����������������ںϳ��ӷ����ʻ��������ʻ������ӷ������ʲ����ڲ�������ˣ����ʻ������ӷ����ϸ��¶�����T2ʱ���ʻ������ֽ⣬�����۲������Ҷˣ�һ����̼ѭ�����ã�
���� ���⿼���������仯�������ʵķ����жϺͷ�Ӧ����ļ����жϣ�Ԫ���غ㡢Ԫ�ػ��ϼ��ǽ���ؼ�����Ŀ�Ѷ��еȣ�

 ���㼤�������100�ִ��Ծ�ϵ�д�
���㼤�������100�ִ��Ծ�ϵ�д�| A�� | 17.1g | B�� | 8.55g | C�� | 34.2g | D�� | 3.4g |
| A�� | 39g | B�� | 59g | C�� | 78g | D�� | 97g |
| A�� | ���³�ѹ�£�17g����-14CH3��������������Ϊ9NA | |
| B�� | 1 L 0.2 mol•L-1��������Һ�к��е�SO42-��Ϊ0.2NA | |
| C�� | 0.1mol N2��������H2��Ӧ��ת�Ƶĵ�����Ϊ0.6NA | |
| D�� | �ö��Ե缫���1L0.1mol•L-1 CuCl2��Һ������0.2NA������ͨ��ʱ��������6.4gͭ |
| A�� | ��ѧ��Ӧ���е�Խ�죬��ѧ��Ӧ����Խ���� | |
| B�� | ij��Ӧ�У���2min��BŨ�ȼ���0.6mol•L-1����2minʱ��B��ʾ�ķ�Ӧ����Ϊ0.6mol•L-1•min | |
| C�� | ��ѧ��Ӧ�е������仯���ǻ�ѧ��ת�������� | |
| D�� | ԭ��صķ�Ӧһ����������ԭ��Ӧ |
| A�� | CH4 | B�� | NaCl | C�� | H2SO4 | D�� | O2 |