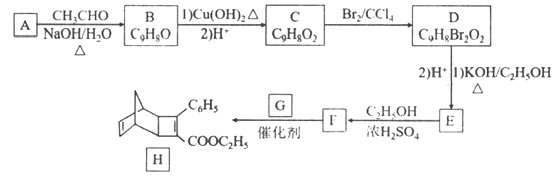
��Ŀ����
����Ŀ����Ԫ������Ȼ������Ҫ��������������Ҫ�ɷ�ΪAl2O3��������Fe2O3��FeO��SiO2���С���ҵ�����������Ʊ�����ij�ֻ�����Ĺ����������¡�
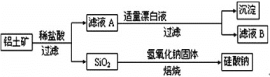
(1)����ҺA�м���Ư��Һ��������ҺB�����ԡ�
����ҺA�м���Ư��Һ��Ŀ���ǣ�________________________�������ӷ���ʽ��ʾ����
������ҺB�е���Ԫ���Գ�����ʽ��������ѡ�õ�����Լ�Ϊ������ţ�___________��
A������������Һ B��������Һ C����ˮ D��������̼
������Ӧ�����ӷ���ʽΪ____________
(2)������ҺB���Ƿ���Fe2+��ѡ����Լ�������Ϊ��_______________________________________
(3)������������������������������Һ�����Ӧ�����ӷ���ʽΪ________________________________________��
���𰸡�2Fe2++ClO-+5H2O=2Fe��OH��3��+C1-+4H+ C Al3++3NH3.H2O=Al(OH)3��+3NH4+ ���軯�ز�����ɫ���������Ը��������ɫ��ȥ SiO2+2OH- = SiO32-+ H2O��Al2O3 + 2 OH- = 2 AlO2- + H2O
��������
��������ϡ���ᷴӦ�����˺�õ�SiO2����ҺA����ҺA�к���Al3+��Fe3+��Fe2+,����ҺA�м���Ư��Һ��Ŀ��������������������ҺB�����ԣ�����������Ӿ���������,������������Ϊ����������Һ���γ���������������ȥ��������ҺB������,����Al3+�����ݴ˷������н��
��1����ҺA�м���Ư��Һ��Ŀ������������������������Ӿ���������������������Ϊ����������Һ���γ���������������ȥ�����ӷ���ʽΪ2Fe2++ClO-+5H2O=2Fe��OH��3��+C1-+4H+����Ԫ�ؼ�Al3+�Գ�����ʽ������Ҳ��������Al��OH��3����ѡ�õ�����Լ�Ϊ��ˮ�����Լӹ�����ˮ�����������ӷ���Ϊ��Al3++3NH3.H2O=Al(OH)3��+3NH4+��
�ʴ�Ϊ2Fe2++ClO-+5H2O=2Fe��OH��3��+C1-+4H+ ��C �� Al3++3NH3.H2O=Al(OH)3��+3NH4+
��2����ҺB���Ƿ���Fe2+�ķ���Ϊ�������軯�ز�����ɫ������������Ը��������ɫ��ȥ����û����ɫ��������ɫ����ȥ��˵����ҺB�в�����Fe2+��
�ʴ�Ϊ�� ���軯�ز�����ɫ���������Ը��������ɫ��ȥ��
��3����������Ҫ�ɷ�ΪAl2O3��������Fe2O3��FeO��SiO2������������������Һ����Al2O3��SiO2������Ӧ���ܽ�Al2O3��SiO2����Ӧ����ʽΪSiO2 +2OH- = SiO32- + H2O��Al2O3 + 2 OH- = 2 AlO2- + H2O��
�ʴ�Ϊ�� SiO2+2OH-= SiO32- + H2O�� Al2O3 + 2 OH- = 2 AlO2- + H2O��

 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д�