��Ŀ����
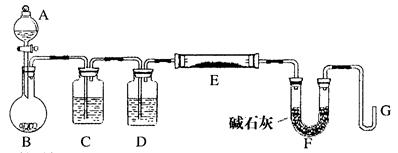
(8��)��֪��Cu+2H2SO4(Ũ)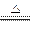 CuSO4+SO2��+2H2O����ͼ��ͭ��Ũ���ᷴӦ��ʵ��װ��ͼ����ش��������⡣
CuSO4+SO2��+2H2O����ͼ��ͭ��Ũ���ᷴӦ��ʵ��װ��ͼ����ش��������⡣
��1��д����Ţٵ����������ƣ� ������ ������
��2������������ͨ��Ʒ����Һ�е�����Ϊ�� �� ��
��3��ͼ�������н�������������Һ����������_________________________ ��������
��4����Ӧ������Ũ���ᱻ���ģ���ϡ����ͭ����ϡ���ᷴӦ����˷�Ӧ��ϣ��Թ���һ����������ʣ�ࡣ����ҩƷ��������֤����Ӧ���������Һ��ȷ���������________(����ĸ)��
a������ b��BaCl2��Һ c������ d��Na2CO3��Һ
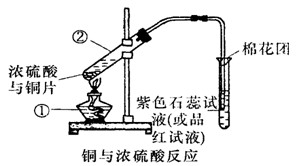
 CuSO4+SO2��+2H2O����ͼ��ͭ��Ũ���ᷴӦ��ʵ��װ��ͼ����ش��������⡣
CuSO4+SO2��+2H2O����ͼ��ͭ��Ũ���ᷴӦ��ʵ��װ��ͼ����ش��������⡣
��1��д����Ţٵ����������ƣ� ������ ������
��2������������ͨ��Ʒ����Һ�е�����Ϊ�� �� ��
��3��ͼ�������н�������������Һ����������_________________________ ��������
��4����Ӧ������Ũ���ᱻ���ģ���ϡ����ͭ����ϡ���ᷴӦ����˷�Ӧ��ϣ��Թ���һ����������ʣ�ࡣ����ҩƷ��������֤����Ӧ���������Һ��ȷ���������________(����ĸ)��
a������ b��BaCl2��Һ c������ d��Na2CO3��Һ
С��1:�ƾ���
С��2:Ʒ����Һ��ɫ
С��3:����SO2β��
��4:ad
��

��ϰ��ϵ�д�
 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�
�����Ŀ