��Ŀ����
����˵������ȷ���� �� ��
| A�������ʵ���Ũ��NaCl��CH3COONa��Һ�������ϣ� c(Cl-)>c(Na+)>c(CH3COO-)>c(OH-)>c(H+) |
| B��25��ʱ����10mL���ᣨpHa����100mLBa��OH��2��Һ��pHn����Ϻ�ǡ���кͣ���pHa+pHn=13 |
| C�������ʵ���Ũ�ȵ��������������Һ�������ϣ�c��SO42-��+c��OH-��=c��H+��+c��CH3COOH�� |
D����ˮ�м�ˮϡ�ͣ� ��С ��С |
B
��

��ϰ��ϵ�д�
 ��ʦ����ָ���ο�ʱϵ�д�
��ʦ����ָ���ο�ʱϵ�д�
�����Ŀ
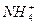 )=c(Na+)=c(OH-)��c(NH3��H2O)
)=c(Na+)=c(OH-)��c(NH3��H2O) +) + c(H+) = c(S2�D) + c(HS�D) + c(OH�D)
+) + c(H+) = c(S2�D) + c(HS�D) + c(OH�D)