��Ŀ����
��֪�ס��ҡ�����Ϊ�������壬���м��ڿ����к�����࣬������ͬ�������ܶ���С�����д̼�����ζ����һ�����������ĸ�ԭ�ӹ��ɡ�
��1��ʵ�����п�����ͼA��Bװ������Ӧ��ҩƷ�Ƶñ���
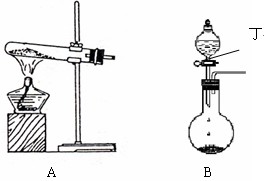
�� A���Թ��ڷ�Ӧ�Ļ�ѧ����ʽ�� ��
�� B�з�Һ©����ʢ�ŵ����ʶ��� ��Բ����ƿ�ڵ������� �����������ƣ�
��2����ҵ�Ͻ������ڸ��¡���ѹ����������������ȡ������ͼ�Ǽ��ҷ�Ӧ�����������仯ͼ��
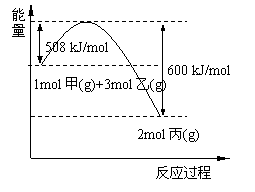 |
�÷�Ӧ���Ȼ�ѧ����ʽ�� ��
��3������������ȼ�յķ�Ӧ���û���Ӧ���÷�Ӧ�Ļ�ѧ����ʽ�� ��
��4���� ������CO2����ͨ�뱥��ʳ��ˮ����̼�����ƾ�����������Ӧ�����ӷ���ʽ�� ��
��Ϊ����֤��������Ȳ���NH4HCO3��Ҳ����NaCl����NaHCO3�����ʵ�鷽�����£�������в���ʵ�鱨�棺
ʵ����� | ʵ������ | ���� | ��Ӧ�����ӷ���ʽ |
ȡ�����������Թ��У���ּ��� | �Թ����й���ʣ�� |
|
|
| ����ȫ���ܽ⣬�����ݲ��� |
|
|
��5�����ã�1���е�A��ȡ������������ƿͨ���������ռ���(��״��)��Ȼ�������Ȫʵ�顣��ˮ���뵽��ƿ�����3/5ʱ��Һ�治����������ʱ�����ر�ֹˮ�У���ƿ����Һ�����ʵ����ʵ���Ũ���� mol/L����ȷ0.001����


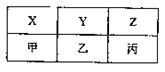
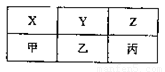
 ��4��X��Y��Z���ס��ҡ�������A��B��C�ֱ���D��
��4��X��Y��Z���ס��ҡ�������A��B��C�ֱ���D��