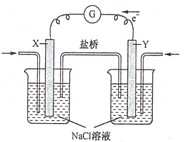
��Ŀ����
����Ŀ����һƿ��ɫ�������Һ�����п��ܺ�NH4+��K+��Na+��Mg2+��H+��Cu2+��CO32-��I-�е�һ�ֻ��֣�ȡ����Һ��������ʵ�飺
����PH��ֽ���飬������Һ��ǿ����
��ȡ������Һ������������CCl4���������Ƶ���ˮ����CCl4������ɫ
����ȡ������Һ����μ���ϡNaOH��Һ��ʹ��Һ��������ת��Ϊ���ԣ��ڵμӹ����м��μ���Ϻ���Һ�о���������
�ܽ��۵õ��ļ�����Һ���ȣ�������ų�����������ʹʪ��ĺ�ɫʯ����ֽ������
��������ʵ����ʵȷ�����ش�
(1)����Һ�У��϶����ڵ���___________���϶������ڵ�������__________________��
(2)д��ʵ����е����ӷ�Ӧ����ʽ________________________��
(3)����ȷ���Ƿ���ڵ�������__________________________��
���𰸡�I-��NH4+��H+ CO32- ��Mg2+ ��Cu2+ 2I- + Cl2��I2 + 2Cl- K+��Na+
��������
����ʵ���������Һ��ǿ���ԣ�˵����Һ�п϶�����H+����H+��CO32-������Ӧ�����ܹ��棬˵����Һ�п϶�������CO32-������ʵ�������CCl4����Ϻ�ɫ��˵����I2���ɣ���������I-�����������������ģ��Ӷ�˵����Һ�к���I-������ʵ���������Һ��������Ϊ���ԣ��ڵμӹ����к͵μ���Ϻ���Һ��������������Mg2+��Cu2+����Ӧ����������˵����Һ�п϶�������Mg2+��Cu2+������ʵ�����������������ʹʪ��ĺ�ɫʯ����ֽ������������Ϊ�������������� NH4++OH-![]() NH3��+H2O�������Һ�п϶�����NH4+���������������ṩ�������п϶����е�����Ϊ��H+��I-��NH4+���϶������е�����Ϊ��Mg2+��Cu2+��CO32-��������ȷ��������Ϊ��K+��Na+��Ҫȷ��K+��Na+������Ҫͨ����ɫ��Ӧ����ɣ�������ɫ��Ӧʵ�飬�л�ɫ������Na+����ɫ������Na+������ɫ�ܲ����۲죬����ɫ������K+������ɫ������K+��
NH3��+H2O�������Һ�п϶�����NH4+���������������ṩ�������п϶����е�����Ϊ��H+��I-��NH4+���϶������е�����Ϊ��Mg2+��Cu2+��CO32-��������ȷ��������Ϊ��K+��Na+��Ҫȷ��K+��Na+������Ҫͨ����ɫ��Ӧ����ɣ�������ɫ��Ӧʵ�飬�л�ɫ������Na+����ɫ������Na+������ɫ�ܲ����۲죬����ɫ������K+������ɫ������K+��
(1)�ɷ�����֪������Һ�У��϶����ڵ���I-��NH4+��H+���϶������ڵ�������CO32- ��Mg2+ ��Cu2+��
(2) ʵ�������CCl4����Ϻ�ɫ��˵����I2���ɣ���������I-�����������������ģ����ӷ�Ӧ����ʽΪ��2I- + Cl2��I2 + 2Cl-��
(3) ����ȷ���Ƿ���ڵ�������K+��Na+��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ����(B)���仯�����ڻ�ѧ��ҵ���������;����ش���������:
(1)���⻯��(NaBH4)�������Ҫ�����
��NaBH4��B�Ļ��ϼ�Ϊ_______________��
�ڹ�ҵ�Ͽ������������[B(OCH3)3]���⻯��(NaH)��Ӧ�Ʊ�NaBH4��Ӧ�����ֲ���Ϊ�״���(CH3ONa)���÷�Ӧ�Ļ�ѧ����ʽΪ______________________��
��NaBH4��ˮ��Ӧ����NaBO2��H2���÷�Ӧ���ɵ����������뻹ԭ��������ʵ���֮��Ϊ____________________��
(2)��ҵ���������(��Ҫ�ɷ�ΪMg2B2O5H2O,��������Fe2O3��FeO��CaO��Al2O3��SiO2��)Ϊԭ���Ʊ�����B�Ĺ���������ͼ��ʾ:
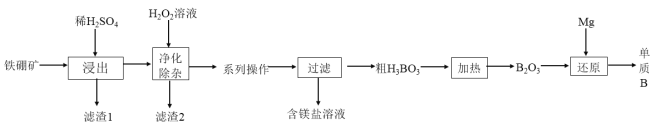
��֪:��ؽ������������������������pH����������:
�������� | Fe3+ | Al3+ | Fe2+ | Mg2+ |
��ʼ������pH | 2.7 | 3.7 | 5.8 | 9.6 |
��ȫ������pH | 3.7 | 4.7 | 8.8 | 11 |
��ش���������:
�١�������ʱ.�������ʯ�����Ŀ��Ϊ___________________________��д��Mg2B2O5H2O�����ᷴӦ�Ļ�ѧ����ʽ:____________________��
������1����Ҫ�ɷ�Ϊ_____________________________��
�ۡ��������ӡ�ʱ���ȼ�H2O2��Һ,��Ŀ��Ϊ_______________��Ȼ���ٵ�����Һ��pH��5,��Ŀ����___________________________________��
���ƵõĴ�����һ��������������BI3 , BI3���ȷֽ���Եõ������ĵ������ֽ�0.025 g�����Ƴɵ�BI3��ȫ�ֽ�,���ɵ�I2��0.30 molL-1 Na2S2O3��Һ�ζ�(I2 +2S2O32-=2I-+ S4O6 2-)���յ㣬���� 18.00 mLNa2S2O3��Һ:ʢװNa2S2O3��ҺӦ��_____________���ʽ����ʽ��)�ζ��ܣ��ô�����Ʒ�Ĵ���Ϊ________________��
����Ŀ����1����֪����ʱ��0.1 mol/L������ˮ����0.1%�������룬�����Һ��pH��__������ĵ���ƽ�ⳣ��K��__��
��2�������������μ�ˮ����Һ�����������ˮ������仯����ͼ��ʾ��
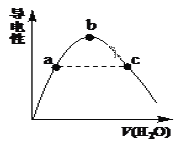
�� a��b��c������Һ��CH3COOH�ĵ���̶��ɴ�С��˳����_____________��
�� a��c�����Ӧ����Һ�ֱ����հ�����������Һ����pH��Ϊ7��25��ʱ������a ����Һ�е�c(CH3COO��) __c����Һ�е�c(NH4+)��(������������������������)
��3����֪25��ʱ����������ʵĵ���ƽ�ⳣ�����������ʾ���ش��������⣺
��ѧʽ | CH3COOH | H2CO3 | HClO |
����ƽ�ⳣ�� | Ka=1.8 | Ka1=4.3 | Ka=3.0 |
�����ʵ���Ũ�Ⱦ�Ϊ0.1mol/L��������Һ��pH��С�������е�˳����______(�ñ����д)��
a��CH3COONa b��Na2CO3 c��NaClO d��NaHCO3
��д�������������Һ��ͨ������������̼�����ӷ���ʽ��__________��