��Ŀ����
15��25��ʱ���й�����ĵ��볣�����£�| ����Ļ�ѧʽ | CH3COOH | HCN | H2S |
| ���볣����25�棩 | 1.8��10-5 | 4.9��10-10 | K1=1.3��10-7 K2=7.1��10-15 |
| A�� | �����ʵ���Ũ�ȵĸ���ҺpH��ϵΪ��pH��CH3COONa����pH��Na2S����pH��NaCN�� | |
| B�� | a mol•L-1HCN��Һ��b��mol•L-1NaOH��Һ�������ϣ�������Һ��c��Na+����c��CN-������aһ��С�ڻ����b | |
| C�� | HCN��Na2S��Һһ�����ܷ�����Ӧ | |
| D�� | NaHS��Na2S�Ļ����Һ�У�һ������c��Na+��+c��H+��=c��OH-��+c��HS-��+2c��S2-�� |
���� A������ƽ�ⳣ��Խ������Խǿ������̶�Խ��
B����Ũ��ʱ����NaCN��CN-����ˮ�⣻
C��HCN����������H2S����HS-ǿ��
D�����ݵ���غ������
��� �⣺A���ɱ����е����ݿ�֪���������ƽ�ⳣ�����������ǿ��������Խǿ���ε�ˮ��Խ�������Ե����ʵ���Ũ����Һ��pH��ϵΪpH��Na2S����pH��NaCN����pH��CH3COONa������A����
B�������ʵ���ʱ����NaCN��CN-����ˮ�⣬��C��Na+����c��CN-��������a mol•L-1HCN��Һ��b mol•L-1NaOH��Һ�������Ϻ�������Һ�У�c��Na+����c��CN-����a�п����Դ���b����B����
C��HCN����������H2S����HS-ǿ������HCN��Na2S��Һ�ܷ�����Ӧ����C����
D��NaHS��Na2S�Ļ����Һ�д��ڵ���غ㣬����һ������c��Na+��+c��H+��=c��OH-��+c��HS-��+2c��S2-������D��ȷ��
��ѡD��
���� ���⿼����롢ˮ�⼰��Һ������Ũ�ȵĹ�ϵ���ۺ��Խ�ǿ����Ŀ�Ѷ��еȣ�ע����������ݵ�Ӧ�������

��ϰ��ϵ�д�
�����Ŀ
6����ѧ���ѧ����������ᡢ����������أ������й�˵���в���ȷ���ǣ�������
| A�� | ����Һ���Լ�Һ������Ҫ���ȣ���������������� | |
| B�� | ��������ʱ������һЩʳ���Ͼƻ���㣬����Ϊ������������ | |
| C�� | ���ع��͡������ӹ���������������Ʒ��� | |
| D�� | �������﷽���ѳ�������ˮ�еĵ����ף���ֹˮ�帻Ӫ���� |
3������CuO��Fe2O3��ɵĻ����a g�������м���2mol•L-1��������Һ100mL��ǡ����ȫ��Ӧ������a g�û����������H2�м��ȣ�ʹ���ַ�Ӧ����ȴ��ʣ���������Ϊ��������
| A�� | 1.6a g | B�� | ��a-3.2��g | C�� | ��a-1.6��g | D�� | 1.6 g |
10��������Ԫ��A��B��C��D��ԭ��������������ԭ�Ӱ뾶rC��rD��rB��rA��Bԭ�����������������ڲ����������3����Dԭ�ӵĺ˵��������A��Cԭ�Ӻ˵����֮�ͣ�A��Cͬ���壮����˵����ȷ���ǣ�������
| A�� | B�ĵ��ʲ�����ͬ�������� | |
| B�� | ����D�Ż�ʱ�����ö�����̼�������� | |
| C�� | ������A2B2��C2B2��ֻ�������� | |
| D�� | A��B��C��ɵĻ����25��ʱ����ҺŨ��Ϊ0.1mol/L������ˮ�������OH-Ϊ10-13mol/L |
20���±���Ԫ�����ڱ���һ���֣���ش��������⣺
��1��д������Ԫ�ط��ţ���N����Si
��2������ЩԪ�ص�����������ˮ�����У�������ǿ����HClO4���ѧʽ��
��3������ЩԪ���У�ԭ�Ӱ뾶������Na����Ԫ�ط��ţ�
| �� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| �� | �� | �� | ||||||
| �� | �� | �� | �� | �� | �� | �� | �� |
��2������ЩԪ�ص�����������ˮ�����У�������ǿ����HClO4���ѧʽ��
��3������ЩԪ���У�ԭ�Ӱ뾶������Na����Ԫ�ط��ţ�
7�����и����������ʵ��������� ��NA��ʾ�����ӵ���������������
| A�� | 2NA��H2O | B�� | ���³�ѹ�£�48g O2 | ||
| C�� | ��״���£�22.4L H2 | D�� | 0.5mol CO2 |
20����ѧ�ڹ�ũҵ�������ճ������ж�������Ҫ��Ӧ�ã�������������ȷ���ǣ�������
| A�� | ����ʹ�ã�NH4��2SO4���ʻ�ʹ�����ữ��������ʹ������[CO��NH2��2]��ʹ����� | |
| B�� | ��¯ˮ���к��е�CaSO4��������Na2CO3��Һ�������������ȥ | |
| C�� | ���Ͻ�Ĵ���ʹ�ù鹦���������ý�̿�Ȼ�ԭ�����������л�ȡ������ | |
| D�� | ������Գ��⡱�����ȵĴ�����Һȥ���ۡ����������˻�ѧ�仯 |
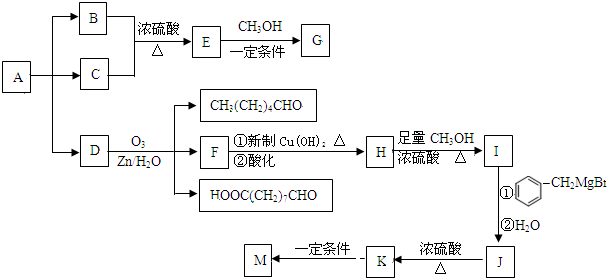
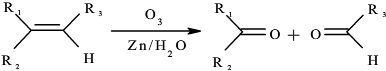
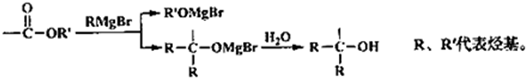
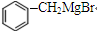 ��
�� +2CH3OMgBr��
+2CH3OMgBr��
 ���÷�Ӧ������Ϊ������Ӧ��
���÷�Ӧ������Ϊ������Ӧ��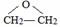 ��
��