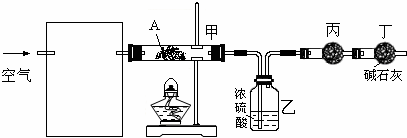
��Ŀ����
A��B��D��E��F��GΪ������Ԫ�أ���ԭ���������ε�����A��Fͬ���壬E��Gͬ���壮A�������ǽ���Ԫ�ػ���ʱ���γɹ��ۼ���F�������ǽ���Ԫ�ػ���ʱ���γ����Ӽ�����F+������E2-���Ӻ�������Ų���ͬ��������Ԫ����ɵ�����BE��D2������ͬ�ĵ���������ش��������⣺
��1��F�
��2��G�����ӽṹʾ��ͼ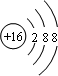
 ��
��
��3���õ���ʽ��ʾD2���γɹ���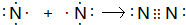
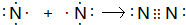 ��D���⻯����������������Ӧ��ˮ���ﷴӦ������ˮ��Һ��
��D���⻯����������������Ӧ��ˮ���ﷴӦ������ˮ��Һ��
��4��B�������
��1��F�
��
��
���ڣ���A
��A
�壻��2��G�����ӽṹʾ��ͼ


��3���õ���ʽ��ʾD2���γɹ���
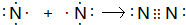
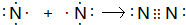
��
��
�ԣ������ӷ���ʽ��ʾԭ��NH4++H2O?NH3��H2O+H+
NH4++H2O?NH3��H2O+H+
����4��B�������
+4
+4
����A��B��ɵĻ������У���A����ߵ���̬���ʵĻ�ѧʽ��CH4
CH4
����D2ʽ����ȵ����ʵĻ�ѧʽ��C2H4
C2H4
�����еĻ�ѧ�������Լ����Ǽ��Լ�
���Լ����Ǽ��Լ�
����д������ѧ�����ͣ���������A��B��D��E��F��GΪ������Ԫ�أ���ԭ���������ε�����A��Fͬ���壬F�����γ�+1��F+���ӣ����ߴ��ڢ�A�壬��A��F��ԭ������֮���֪��FΪNaԪ�أ�
A�������ǽ���Ԫ�ػ���ʱ���γɹ��ۼ�����AΪ��Ԫ�أ�
F+������E2-���Ӻ�������Ų���ͬ�����Ӻ��������Ϊ10����EΪ��Ԫ�أ�
E��Gͬ���壬��GΪSԪ�أ�
D�γ�˫ԭ�ӷ��ӣ�Dԭ������С����Ԫ�أ����ڵڶ����ڣ���DΪNԪ�أ�
����BE��D2������ͬ�ĵ���������Bԭ�Ӻ��������Ϊ2��7-8=6����BΪ̼Ԫ�أ��ݴ˽��
A�������ǽ���Ԫ�ػ���ʱ���γɹ��ۼ�����AΪ��Ԫ�أ�
F+������E2-���Ӻ�������Ų���ͬ�����Ӻ��������Ϊ10����EΪ��Ԫ�أ�
E��Gͬ���壬��GΪSԪ�أ�
D�γ�˫ԭ�ӷ��ӣ�Dԭ������С����Ԫ�أ����ڵڶ����ڣ���DΪNԪ�أ�
����BE��D2������ͬ�ĵ���������Bԭ�Ӻ��������Ϊ2��7-8=6����BΪ̼Ԫ�أ��ݴ˽��
����⣺A��B��D��E��F��GΪ������Ԫ�أ���ԭ���������ε�����A��Fͬ���壬F�����γ�+1��F+���ӣ����ߴ��ڢ�A�壬��A��F��ԭ������֮���֪��FΪNaԪ�أ�
A�������ǽ���Ԫ�ػ���ʱ���γɹ��ۼ�����AΪ��Ԫ�أ�
F+������E2-���Ӻ�������Ų���ͬ�����Ӻ��������Ϊ10����EΪ��Ԫ�أ�
E��Gͬ���壬��GΪSԪ�أ�
D�γ�˫ԭ�ӷ��ӣ�Dԭ������С����Ԫ�أ����ڵڶ����ڣ���DΪNԪ�أ�
����BE��D2������ͬ�ĵ���������Bԭ�Ӻ��������Ϊ2��7-8=6����BΪ̼Ԫ�أ�
��1��FΪNaԪ�أ�λ�ڵ������ڵڢ�A�壬
�ʴ�Ϊ��������A��
��2��GΪ��Ԫ�أ�S2-���ӽṹʾ��ͼΪ ��
��
�ʴ�Ϊ�� ��
��
��3���õ���ʽ��ʾN2���γɹ���Ϊ��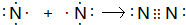 ��
��
NԪ�ص��⻯����������������Ӧ��ˮ���ﷴӦΪNH4NO3����Һ��笠�����ˮ��NH4++H2O?NH3?H2O+H+��
���ƻ�ˮ�ĵ���ƽ�⣬��Һ�����ԣ�
�ʴ�Ϊ��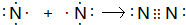 ���NH4++H2O?NH3?H2O+H+��
���NH4++H2O?NH3?H2O+H+��
��4��BΪ̼Ԫ�أ��������+4����H��C��ɵĻ������У���������ߵ���̬���ʵĻ�ѧʽ��CH4����N2ʽ����ȵ����ʵĻ�ѧʽ��C2H4�����еĻ�ѧ���м��Լ����Ǽ��Լ���
�ʴ�Ϊ��+4��CH4��C2H4�����Լ����Ǽ��Լ���
A�������ǽ���Ԫ�ػ���ʱ���γɹ��ۼ�����AΪ��Ԫ�أ�
F+������E2-���Ӻ�������Ų���ͬ�����Ӻ��������Ϊ10����EΪ��Ԫ�أ�
E��Gͬ���壬��GΪSԪ�أ�
D�γ�˫ԭ�ӷ��ӣ�Dԭ������С����Ԫ�أ����ڵڶ����ڣ���DΪNԪ�أ�
����BE��D2������ͬ�ĵ���������Bԭ�Ӻ��������Ϊ2��7-8=6����BΪ̼Ԫ�أ�
��1��FΪNaԪ�أ�λ�ڵ������ڵڢ�A�壬
�ʴ�Ϊ��������A��
��2��GΪ��Ԫ�أ�S2-���ӽṹʾ��ͼΪ
 ��
���ʴ�Ϊ��
 ��
����3���õ���ʽ��ʾN2���γɹ���Ϊ��
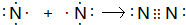 ��
��NԪ�ص��⻯����������������Ӧ��ˮ���ﷴӦΪNH4NO3����Һ��笠�����ˮ��NH4++H2O?NH3?H2O+H+��
���ƻ�ˮ�ĵ���ƽ�⣬��Һ�����ԣ�
�ʴ�Ϊ��
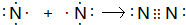 ���NH4++H2O?NH3?H2O+H+��
���NH4++H2O?NH3?H2O+H+����4��BΪ̼Ԫ�أ��������+4����H��C��ɵĻ������У���������ߵ���̬���ʵĻ�ѧʽ��CH4����N2ʽ����ȵ����ʵĻ�ѧʽ��C2H4�����еĻ�ѧ���м��Լ����Ǽ��Լ���
�ʴ�Ϊ��+4��CH4��C2H4�����Լ����Ǽ��Լ���
���������⿼��ṹ����λ�ù�ϵ���漰���û�ѧ�������ˮ�⡢��ѧ����Ԫ�ػ�����֪ʶ�ȣ�Ԫ�ص��ƶ��ǽ��Ĺؼ����Ѷ��еȣ�

��ϰ��ϵ�д�
 �Ͻ�ƽ���Ȿϵ�д�
�Ͻ�ƽ���Ȿϵ�д� ����ѧ��Ӧ�����ϵ�д�
����ѧ��Ӧ�����ϵ�д�
�����Ŀ

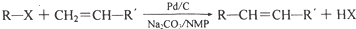
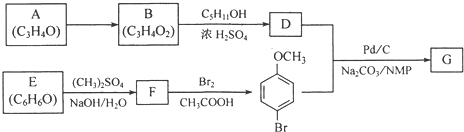

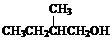
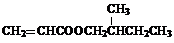 +H2O
+H2O
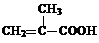

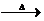 CH3CHO+H2O+Cu
CH3CHO+H2O+Cu