��Ŀ����
��2011?������ģ����֪A��B��D��E��F��GΪ��ѧ��ѧ�г����Ļ��������֮������ͼ1��ʾ��ת����ϵ����Ӧ���������ֲ�������ȥ����AΪ��ɫ��ĩ����H��C��O��Cu����Ԫ�أ������£�DΪ��ɫ��ζ���壬BΪ��ɫ��ĩ��E���ӽṹ�к���ȩ����
��ش��������⣺
��1��D��G��Ӧ�Ļ�ѧ����ʽ
��2��F��һ�����еĹ���������
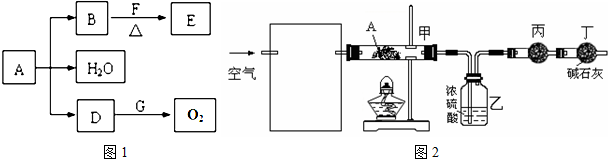
��3��ij����С�����������ʵ��װ�ã�ͨ���ⶨװ�ü������Լ��������仯��̽��A�Ļ�ѧʽ��
��Ϊʹ����ȷ�����貹��װ�ã������ڷ����ڻ��װ��ͼ��д���Լ����ƣ�
����װ���й��������Ŀ����
������ж�A����ȫ�ֽ⣿
��ʵ���ó��������ݣ�A���Ⱥ���ȫ�ֽ⣬������8.0g��Ϊ6.0g��װ��������0.9g��д��A�Ļ�ѧʽ����ʾΪ��ʽ�Σ���

��ش��������⣺
��1��D��G��Ӧ�Ļ�ѧ����ʽ
2Na2O2+2CO2=2Na2CO3+O2
2Na2O2+2CO2=2Na2CO3+O2
����2��F��һ�����еĹ���������
�ǻ�
�ǻ�
����3��ij����С�����������ʵ��װ�ã�ͨ���ⶨװ�ü������Լ��������仯��̽��A�Ļ�ѧʽ��
��Ϊʹ����ȷ�����貹��װ�ã������ڷ����ڻ��װ��ͼ��д���Լ����ƣ�
����װ���й��������Ŀ����
��A�ֽ������ˮ��������ʢ��Ũ�����ϴ��ƿ��
��A�ֽ������ˮ��������ʢ��Ũ�����ϴ��ƿ��
����װ����ҩƷ����������ˮ����ͭ
��ˮ����ͭ
��ʵ��ʱ����ҩƷδ�����Ա仯��֤��A�ֽ������ˮ����ȫ����Ũ��������
A�ֽ������ˮ����ȫ����Ũ��������
��������ж�A����ȫ�ֽ⣿
�������μ��ȡ���������ȴ��������װ�õ����������������0.1 g��֤��A�ֽ���ȫ
�������μ��ȡ���������ȴ��������װ�õ����������������0.1 g��֤��A�ֽ���ȫ
����ʵ���ó��������ݣ�A���Ⱥ���ȫ�ֽ⣬������8.0g��Ϊ6.0g��װ��������0.9g��д��A�Ļ�ѧʽ����ʾΪ��ʽ�Σ���
Cu3��OH��4CO3��CuCO3?2Cu��OH��2
Cu3��OH��4CO3��CuCO3?2Cu��OH��2
��������AΪ��ɫ��ĩ����H��C��O��Cu����Ԫ��֤��Ϊͭ�̣������£�DΪ��ɫ��ζ��������G��Ӧ���������ж�DΪCO2��BΪ��ɫ��ĩ�ƶ�ΪCuO��F��E��Ϊ�л����֪F��E����Է�������֮�����2��˵��EF��Ӧ��������ͭ�������°Ѵ��ǻ�����Ϊȩ����FΪ����EΪȩ����֪A��B��D��E��F��GΪ��ѧ��ѧ�г����Ļ���������ж�EΪCH3CHO��FΪCH3CH2OH�������ж����ʾͷ����ش����⣮
����⣺AΪ��ɫ��ĩ����H��C��O��Cu����Ԫ��֤��Ϊͭ�̣������£�DΪ��ɫ��ζ��������G��Ӧ���������ж�DΪCO2��BΪ��ɫ��ĩ�ƶ�ΪCuO��F��E��Ϊ�л����֪F��E����Է�������֮�����2��˵��EF��Ӧ��������ͭ�������°Ѵ��ǻ�����Ϊȩ����FΪ����EΪȩ����֪A��B��D��E��F��GΪ��ѧ��ѧ�г����Ļ���������ж�EΪCH3CHO��FΪCH3CH2OH��
��1��D��CO2����G��Na2O2����Ӧ�Ļ�ѧ����Ϊ��2Na2O2+2CO2=2Na2CO3+O2���ʴ�Ϊ��2Na2O2+2CO2=2Na2CO3+O2��
��2��B��CuO����F��C2H5OH���ڼ��������·�Ӧ����E��CH3CHO����ȥ��������Ӧ����Ӧ�Ļ�ѧ����ʽΪ��CH3CH2OH+CuO
CH3CHO+H2O+Cu������F��һ�������ǻ���
�ʴ�Ϊ���ǻ���
��3�����ⶨװ�ü������Լ��������仯��̽��A�Ļ�ѧʽ��
��Ϊʹ����ȷ��Ҫ��ͨ��Ŀ����е�ˮ������ȥ������Ӱ��ⶨ�����װ��Ϊ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
����װ���й��������Ŀ���ǣ���A�ֽ������ˮ��������ʢ��Ũ�����ϴ��ƿ�У���װ����ҩƷ����������ˮ����ͭ�������Ǽ��ˮ�����Ƿ�������ޱ仯֤��ˮ����������A�ֽ������ˮ����ȫ����Ũ�������գ�
�ʴ�Ϊ����A�ֽ������ˮ��������ʢ��Ũ�����ϴ��ƿ�У���ˮ����ͭ��A�ֽ������ˮ����ȫ����Ũ�������գ�
���ж�A����ȫ�ֽ�ķ���Ϊ���������μ��ȡ���������ȴ��������װ�õ����������������0.1 g��֤��A�ֽ���ȫ��
�ʴ�Ϊ���������μ��ȡ���������ȴ��������װ�õ����������������0.1 g��֤��A�ֽ���ȫ��
��ʵ���ó��������ݣ�A���Ⱥ���ȫ�ֽ⣬������8.0g��Ϊ6.0g��װ��������0.9gΪ���ɵ�ˮ�������������������1.1gΪCO2������ԭ���غ㣬8.0gA�к��е���Ԫ�����ʵ���Ϊ
=0.1mol������̼Ԫ�����ʵ���Ϊ
=0.025mol�����ɵ�����ͭ����Ϊ6g��ͭ���ʵ���Ϊ
=0.075mol����Ԫ�ص�����Ϊ8.0g-0.1mol��1g/mol-0.025mol��12g/mol-0.075mol��64g/mol=2.8g����Ԫ�����ʵ���Ϊ���ʵ���Ϊ=
=0.175mol��A��Ԫ��ԭ�����ʵ���֮��Ϊn��Cu����n��C����n��H����n��O��=0.075��0.025��0.1��0.175=3��1��4��7����ѧʽΪCu3H4CO7����Ϸ�Ӧת����֪���ṹ�к�̼�����ʣ��Ϊ��������д����ѧʽΪ��Cu3��OH��4CO3��CuCO3?2Cu��OH��2��
�ʴ�Ϊ��Cu3��OH��4CO3��CuCO3?2Cu��OH��2��
��1��D��CO2����G��Na2O2����Ӧ�Ļ�ѧ����Ϊ��2Na2O2+2CO2=2Na2CO3+O2���ʴ�Ϊ��2Na2O2+2CO2=2Na2CO3+O2��
��2��B��CuO����F��C2H5OH���ڼ��������·�Ӧ����E��CH3CHO����ȥ��������Ӧ����Ӧ�Ļ�ѧ����ʽΪ��CH3CH2OH+CuO
| �� |
�ʴ�Ϊ���ǻ���
��3�����ⶨװ�ü������Լ��������仯��̽��A�Ļ�ѧʽ��
��Ϊʹ����ȷ��Ҫ��ͨ��Ŀ����е�ˮ������ȥ������Ӱ��ⶨ�����װ��Ϊ
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
������װ���й��������Ŀ���ǣ���A�ֽ������ˮ��������ʢ��Ũ�����ϴ��ƿ�У���װ����ҩƷ����������ˮ����ͭ�������Ǽ��ˮ�����Ƿ�������ޱ仯֤��ˮ����������A�ֽ������ˮ����ȫ����Ũ�������գ�
�ʴ�Ϊ����A�ֽ������ˮ��������ʢ��Ũ�����ϴ��ƿ�У���ˮ����ͭ��A�ֽ������ˮ����ȫ����Ũ�������գ�
���ж�A����ȫ�ֽ�ķ���Ϊ���������μ��ȡ���������ȴ��������װ�õ����������������0.1 g��֤��A�ֽ���ȫ��
�ʴ�Ϊ���������μ��ȡ���������ȴ��������װ�õ����������������0.1 g��֤��A�ֽ���ȫ��
��ʵ���ó��������ݣ�A���Ⱥ���ȫ�ֽ⣬������8.0g��Ϊ6.0g��װ��������0.9gΪ���ɵ�ˮ�������������������1.1gΪCO2������ԭ���غ㣬8.0gA�к��е���Ԫ�����ʵ���Ϊ
| 0.9g |
| 18g/mol |
| 1.1g |
| 44g/mol |
| 6g |
| 80g/mol |
| 2.8g |
| 16g/mol |
�ʴ�Ϊ��Cu3��OH��4CO3��CuCO3?2Cu��OH��2��
���������⿼��������ת����ϵ���������ʵ��ۺ�Ӧ�ã�ʵ�鷽������ƺ�ʵ��ⶨ��ȷ��������ѧʽ�ļ����жϣ�ʵ����̵ķ����жϣ���Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ