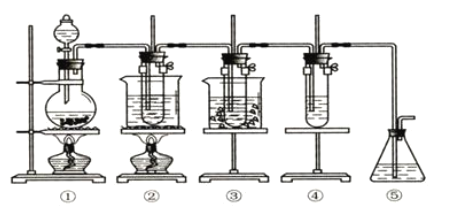
��Ŀ����
����Ŀ�������γ��ڶ���뻯����ش��������⣺
(1)���ڳɼ�ʱ���ܽ�һ��3s���Ӽ�������3d�ܼ����μӳɼ���д���ü���̬ԭ�ӵĺ�������Ų�ʽ__ ��
(2)������һ�ֶ�ά���ϣ�����һ��Ľṹ��ͼ1��ʾ��
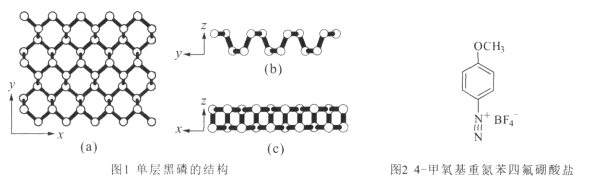
�ٺ�����Pԭ�ӵ��ӻ���ʽΪ _________ ��ÿһ����P�γ���Ԫ���˴���ӣ�ƽ��ÿ���ռ���Ԫ���к��е���ԭ���� ____����
����4-�������ص����ķ������Σ���ͼ2�������������ײ��ϣ����Ա����Ϳ��������ʡ�
���εĹ���Ԫ����C��N��O��F�ĵ縺���ɴ�С˳��Ϊ__��1mol���������Ӻ��еĦҼ�����ĿΪ______ �����������ӵļ��ι�����__��
(3)���ƿ����ȡϡ��Ԫ����(Y)��ij���ƿ�Ľṹ���£�
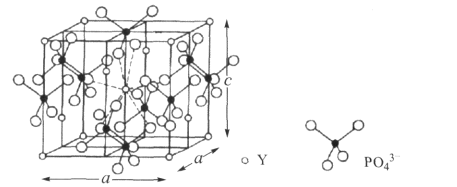
�����ƿ�Ļ�ѧʽΪ__����PO43����Ϊ�ȵ��������������__ ��д���������ӵĻ�ѧʽ������֪��������a= 0.69 nm��c=0.60 nm�������ӵ�����ΪNA��������ƿ���ܶ�Ϊ__g.cm��3���г�����ʽ����
���𰸡�1s22s22p63s13p33d1 sp3 2 F>O>N>C 17NA �������� YPO4 SO42-��ClO4-��BrO4-��IO4-��SiO44- ![]()
��������
(1)��Ϊ15��Ԫ�أ���̬Pԭ�ӵĺ�������Ų�ʽΪ1s22s22p63s23p3�����ڳɼ�ʱ���ܽ�һ��3s���Ӽ�������3d�ܼ����μӳɼ����ü���̬ԭ�ӵĺ�������Ų�ʽΪ1s22s22p63s13p33d1��
(2)�ٺ�����ÿ��Pԭ������������Pԭ���γɹ��ۼ�����һ�Թµ��Ӷԣ�Pԭ�ӵ��ӻ���ʽΪsp3��ÿһ����P�γ���Ԫ���˴���ӣ�ƽ��ÿ���ռ���Ԫ���к��е���ԭ����![]() ����
����
��Ԫ��ԭ�ӵĵõ�������Խǿ����縺��Խ��ԭ�ӵõ���������СΪ��F��O��N��C����縺�Դ�СΪ��F��O��N��C��4-�������ص����ķ��������������к���һ������������һ����̼�������������ĸ�̼���������������̼̼��������̼����������̼�������1mol���������Ӻ��е���������ĿΪ17NA������������BF4-��B�ϵŵ��Ӷ���Ϊ![]() =0���۲���Ӷ���Ϊ0+4=4��BΪsp3�ӻ�����BF4-�Ŀռ乹��Ϊ�������壻
=0���۲���Ӷ���Ϊ0+4=4��BΪsp3�ӻ�����BF4-�Ŀռ乹��Ϊ�������壻
(3)���ݾ�̯�����㣬�����ƿ�һ����������![]() ��Y��
��Y��![]() ��PO43-���ʸ����ƿ�Ļ�ѧʽΪYPO4���ȵ�������ָ�۵���������ԭ�������������ԭ�Ӳ������ڣ���ͬ�ķ��ӡ����ӻ�ԭ���ţ���PO43����Ϊ�ȵ��������������SO42-��ClO4-��BrO4-��IO4-��SiO44-����֪��������a= 0.69 nm��c=0.60 nm�������ӵ�����ΪNA��������ƿ���ܶ�Ϊ
��PO43-���ʸ����ƿ�Ļ�ѧʽΪYPO4���ȵ�������ָ�۵���������ԭ�������������ԭ�Ӳ������ڣ���ͬ�ķ��ӡ����ӻ�ԭ���ţ���PO43����Ϊ�ȵ��������������SO42-��ClO4-��BrO4-��IO4-��SiO44-����֪��������a= 0.69 nm��c=0.60 nm�������ӵ�����ΪNA��������ƿ���ܶ�Ϊ g.cm��3��
g.cm��3��

����Ŀ��25 ��ʱ���������ʵĵ��볣�������ʾ��
��ѧʽ | CH3COOH | H2CO3 |
���볣�� | 1.7��10��5 | K1��4.3��10��7 K2��5.6��10��11 |
��ش��������⣺
(1)��ͬpH��CH3COONa��NaHCO3��Na2CO3Ũ���ɴ�С��˳��Ϊ__________
(2)������0.1mol��L��1��CH3COOH��Һ�ڼ�ˮϡ�����У����б���ʽ������һ����С����________(����ĸ����ͬ)��
A��c(H��) B��c(H��)/c(CH3COOH) C��c(H��)��c(OH��) D��c(H��)/c(OH��)
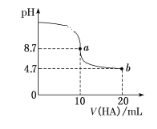
(3)����10mLpH=2��������Һ���������²�����
a.��pH=5������������ϣ���pH=_____________��
b.���������Һ�м���10mL0.02mol/LCH3COONa��Һ����û����Һ�д��ڵ������غ�ʽΪ__________________��
c.���������Һ�м���������Ũ�ȵ�Na2CO3��Һ��������Һ�д��ڵĵ���غ�ʽΪ_______________________��
d. ���й��������Ϊ10mL��pH=2��A(����)��B(CH3COOH)��Һ˵����ȷ����_____ (��д���)��
���������п��Ӧ��ʼ��Ӧʱ������A=B
���������п��Ӧ��п��ȫ�ܽ⣬û��ʣ�ࣩ����Ҫ��ʱ��A>B
�ۼ�ˮϡ��100����pH��С�Ƚϣ�4=A>B>2
�����ʵ���Ũ�ȴ�С�Ƚϣ�A>B
�ݷֱ���10mLpH=12��NaOH��Һ��ַ�Ӧ�����ҺpH��С�Ƚϣ�A<B
e. ���ñ�HCl��Һ�ζ���ˮ��Ӧѡ��________ָʾ�������в����ᵼ�²ⶨ���ƫ�ߵ���___��
A��δ��HCl����Һ��ϴ�ζ���
B���ζ�ǰ��ƿ��������ˮ
C���ζ�ǰ�ζ��ܼ��첿�������ݣ��ζ���������ʧ
D���۲����ʱ���ζ�ǰ���ӣ��ζ�����