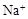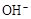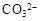��Ŀ����
���и���������ָ����Һ�У��ܴ����������
��������Һ�У�Fe3+��Al3+��NO3����I����Cl��
��pH=11����Һ�У�CO32����Na+��AlO2����NO3����SO32��
����ˮ�����c(H+)=10��12mol��L��1����Һ�У�Cl����K+��NO3����NH4+��S2O32��
�ܼ������ָʾ�����Ժ�ɫ����Һ�У�Mg2+��NH4+��Cl����K+��SO42��
��������Һ�У�Fe3+��Al3+��NO3����I����Cl��
��pH=11����Һ�У�CO32����Na+��AlO2����NO3����SO32��
����ˮ�����c(H+)=10��12mol��L��1����Һ�У�Cl����K+��NO3����NH4+��S2O32��
�ܼ������ָʾ�����Ժ�ɫ����Һ�У�Mg2+��NH4+��Cl����K+��SO42��
| A���٢� | B���٢� | C���ڢ� | D���ۢ� |
C
�����������Fe3+��I-�����棬���ڿ��Դ������ڣ���ȷ���۸���Һ����������Ҳ�����Ǽ��ԣ������ԣ���S2O32-���������ڣ����ܼ����Ժ�ɫ����ҺΪ���ԣ����ӿ��Դ������棬��ȷ��

��ϰ��ϵ�д�
�����Ŀ
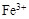 ��
��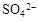 ��
�� ��X�������������ʵ���֮��
��X�������������ʵ���֮�� 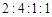 ����������ͬһ��Һ�У���X
����������ͬһ��Һ�У���X