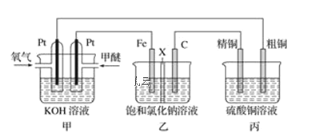
ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩ”–ΜζΈοJ «Έ“ΙζΉ‘÷ς≥…ΙΠ―–ΖΔΒΡ“Μάύ–¬“©Θ§Υϋ τ”ΎθΞάύΘ§Ζ÷Ή”÷–≥ΐ±ΫΜΖΆβΜΙΚ§”–“ΜΗωΈε‘ΣΜΖΓΘΚœ≥…JΒΡ“Μ÷÷¬ΖœΏ»γœ¬ΘΚ
ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©BΒΡΫαΙΙΦρ Ϋ «________________ΓΘCΒΡΫαΙΙΦρ Ϋ «______________ΓΘ
Θ®2Θ©D…ζ≥… EΒΡΜ·―ßΖΫ≥Χ ΫΈΣ_________________ΓΘ
Θ®3Θ©JΒΡΫαΙΙΦρ Ϋ «________________ΓΘ‘Ύ“ΜΕ®ΧθΦΰœ¬Θ§HΉ‘…μΥθΨέ…ζ≥…ΗΏΖ÷Ή”Μ·ΚœΈοΒΡΫαΙΙΦρ Ϋ «_______________ΓΘ
Θ®4Θ©ΗυΨί![]() Θ§XΒΡΖ÷Ή” ΫΈΣ______ΓΘX”–Εύ÷÷Ά§Ζ÷“λΙΙΧεΘ§Τδ÷–¬ζΉψœ¬Ν–ΧθΦΰΒΡΆ§Ζ÷“λΙΙΧεΙ≤”–______÷÷Θ®“―÷ΣΘΚΧΦΧΦ»ΰΦϋΜρΧΦΧΦΥΪΦϋ≤ΜΡή”κτ«Μυ÷±Ϋ”œύΝ§Θ©ΓΘ
Θ§XΒΡΖ÷Ή” ΫΈΣ______ΓΘX”–Εύ÷÷Ά§Ζ÷“λΙΙΧεΘ§Τδ÷–¬ζΉψœ¬Ν–ΧθΦΰΒΡΆ§Ζ÷“λΙΙΧεΙ≤”–______÷÷Θ®“―÷ΣΘΚΧΦΧΦ»ΰΦϋΜρΧΦΧΦΥΪΦϋ≤ΜΡή”κτ«Μυ÷±Ϋ”œύΝ§Θ©ΓΘ
AΘ°≥ΐ±ΫΜΖΆβΈόΤδΥϊΜΖΘ§«“Έό“ΜOΓΣOΓΣΦϋ
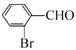
BΘ°Ρή”κFeCl3»ή“ΚΖΔ…ζœ‘…ΪΖ¥”Π
CΘ°±ΫΜΖ…œ“Μ¬»¥ζΈο÷Μ”–ΝΫ÷÷
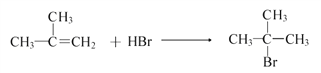
Θ®5Θ©άϊ”ΟΧβ÷––≈œΔΚΆΥυ―ß÷Σ ΕΘ§–¥≥ω“‘ΦΉΆιΚΆΦΉ±Ϋ ΈΣ‘≠ΝœΘ§Κœ≥…![]() ΒΡ¬ΖœΏΝς≥ΧΆΦ(ΤδΥϋ ‘ΦΝΉ‘―Γ)ΘΚ____________________________ΓΘ
ΒΡ¬ΖœΏΝς≥ΧΆΦ(ΤδΥϋ ‘ΦΝΉ‘―Γ)ΘΚ____________________________ΓΘ
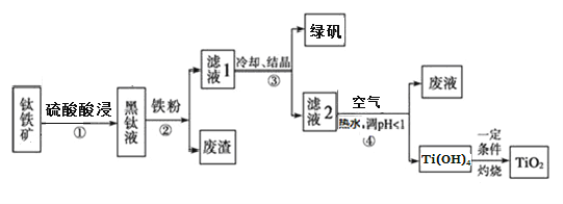
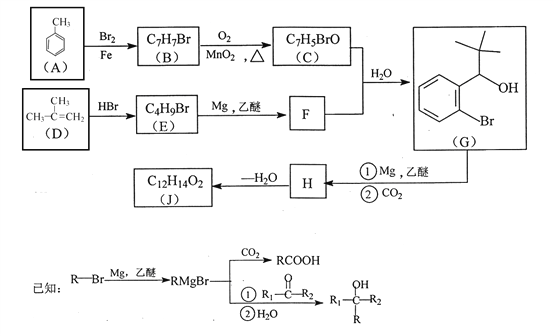
ΓΨ¥πΑΗΓΩ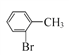
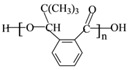 C8H6O3 9
C8H6O3 9 
ΓΨΫβΈωΓΩ
Θ®1Θ©”…A![]() BΒΡΧθΦΰΘ§“‘ΦΑAΓΔGΒΡΫαΙΙΦρ ΫΚΆBΒΡΖ÷Ή” ΫΩ…÷ΣΘ§BΒΡΫαΙΙΦρ ΫΈΣΘΚ
BΒΡΧθΦΰΘ§“‘ΦΑAΓΔGΒΡΫαΙΙΦρ ΫΚΆBΒΡΖ÷Ή” ΫΩ…÷ΣΘ§BΒΡΫαΙΙΦρ ΫΈΣΘΚ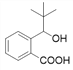 Θ§‘Ό”…B
Θ§‘Ό”…B![]() CΘ§ΗυΨίΖ¥”ΠΧθΦΰΚΆCΒΡΖ÷Ή” ΫΩ…÷ΣΘ§CΈΣ
CΘ§ΗυΨίΖ¥”ΠΧθΦΰΚΆCΒΡΖ÷Ή” ΫΩ…÷ΣΘ§CΈΣ ΘΜ
ΘΜ
Θ®2Θ©D…ζ≥… EΒΡΜ·―ßΖΫ≥Χ ΫΈΣΘΚ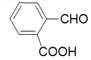 ΘΜ
ΘΜ
Θ®3Θ©”…G![]() H
H![]() JΘ§“‘ΦΑΥυΗχ“―÷ΣΘ§Ω…÷ΣHΈΣ
JΘ§“‘ΦΑΥυΗχ“―÷ΣΘ§Ω…÷ΣHΈΣ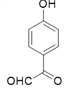 Θ§‘ΌΆ―Υ°ΒΟΒΫJΘ§‘ρJΈΣ
Θ§‘ΌΆ―Υ°ΒΟΒΫJΘ§‘ρJΈΣ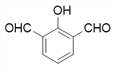 Θ§‘Ύ“ΜΕ®ΧθΦΰœ¬Θ§HΉ‘…μΥθΨέ…ζ≥…ΗΏΖ÷Ή”Μ·ΚœΈοΒΡΫαΙΙΦρ Ϋ «ΘΚ
Θ§‘Ύ“ΜΕ®ΧθΦΰœ¬Θ§HΉ‘…μΥθΨέ…ζ≥…ΗΏΖ÷Ή”Μ·ΚœΈοΒΡΫαΙΙΦρ Ϋ «ΘΚ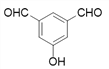 ΘΜ
ΘΜ
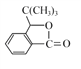
Θ®4Θ©ΗυΨί![]() Θ§XΒΡΫαΙΙΦρ ΫΈΣΘΚ
Θ§XΒΡΫαΙΙΦρ ΫΈΣΘΚ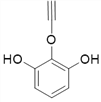 Θ§‘ρΖ÷Ή” ΫΈΣΘΚC8H6O3ΘΜXΒΡΆ§Ζ÷“λΙΙΧε÷–Θ§Ρή”κFeCl3»ή“ΚΖΔ…ζœ‘…ΪΖ¥”ΠΘ§ΥΒΟς”κ±ΫΜΖ÷±Ϋ”œύΝ§ΒΡ”–τ«ΜυΘ§”…“ρΈΣ±ΫΜΖ…œΒΡ“Μ¬»¥ζΈο÷Μ”–“Μ÷÷Θ§«“Έό“ΜOΓΣOΓΣΦϋΘΜΩ…ΡήΒΡ«ιΩω”–
Θ§‘ρΖ÷Ή” ΫΈΣΘΚC8H6O3ΘΜXΒΡΆ§Ζ÷“λΙΙΧε÷–Θ§Ρή”κFeCl3»ή“ΚΖΔ…ζœ‘…ΪΖ¥”ΠΘ§ΥΒΟς”κ±ΫΜΖ÷±Ϋ”œύΝ§ΒΡ”–τ«ΜυΘ§”…“ρΈΣ±ΫΜΖ…œΒΡ“Μ¬»¥ζΈο÷Μ”–“Μ÷÷Θ§«“Έό“ΜOΓΣOΓΣΦϋΘΜΩ…ΡήΒΡ«ιΩω”–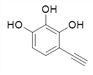
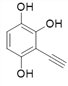
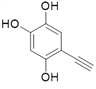
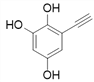
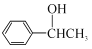
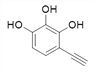
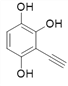
![]()
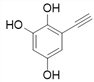
Θ§Ι≤9÷÷ΘΜ
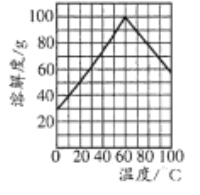
Θ®5Θ©άϊ”ΟΧβ÷––≈œΔΚΆΥυ―ß÷Σ ΕΘ§–¥≥ω“‘ΦΉΆιΚΆΦΉ±Ϋ ΈΣ‘≠ΝœΘ§Κœ≥…![]() ΒΡ¬ΖœΏΝς≥ΧΆΦΘΚ
ΒΡ¬ΖœΏΝς≥ΧΆΦΘΚ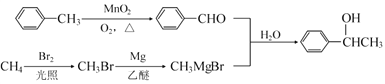 ΓΘ
ΓΘ