��Ŀ����
A��B��Ԫ�����ڱ���ͬһ���ڵ�����Ԫ�أ�Aԭ�Ӻ��е�����������������1��Bԭ�Ӻ�������������������ȣ�Bԭ�����������������ڲ������֮��Ϊ8��A������������ˮ���ﻯѧʽΪH3AO4����ʽ����B������������ˮ����ʽ����ȣ�A����̬�⻯�ﺬ��8.82������ͨ�����㣺
(1)��A��B��Ԫ�ص����ԭ��������
(2)д��A��BԪ�ص����ƺͷ��ţ�
(3)д��A��B����������ˮ�����Ũ��Һ�ֱ���Na2S��Ӧ�Ļ�ѧ����ʽ��
�𰸣�
������
������
|
�⣺(1)��A�����ԭ������Ϊa��B�����ԭ������Ϊb�� ��A������������Ӧˮ����ΪH3AO4�� ��A���Ϊ��5�ۣ���A�壬���⻯��ΪAH3��
�֡�Bԭ�����������������ڲ������֮��Ϊ8����BΪ��A��Ԫ�أ�����ۺ�����Ļ�ѧʽΪH2BO4��M(H2BO4)��M(H3AO4)��98����b��32�� (2)A���������� ��B���������� (3)Na2S��2H2SO4(Ũ)��Na2SO4��S����SO2����2H2O 3Na2S��2H3PO4(Ũ)��2Na3PO4��3H2S�� |

��ϰ��ϵ�д�
 ͬ������ϵ�д�
ͬ������ϵ�д�
�����Ŀ
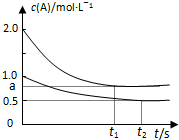 ��ס��������º��ݵ��ܱ������У��ֱ����һ������A��B��������Ӧ��A��g��+B��g��?xC��g����H��0�������������c��A����ʱ��t�ı仯��ͼ��ʾ��
��ס��������º��ݵ��ܱ������У��ֱ����һ������A��B��������Ӧ��A��g��+B��g��?xC��g����H��0�������������c��A����ʱ��t�ı仯��ͼ��ʾ��| ���� | �� | �� |
| �ݻ���L�� | 0.5 | 0.5 |
| ��Ӧ���ȣ�kJ�� | Q1 | Q2 |
| ��Ӧ����ʼ�� | 1 molA 1 molB |
0.5 molA 0.5 molB |
| A��x=1 |
| B��Q1��2Q2 |
| C������������Ϣ������aֵ |
| D�����������������䣬��ʼʱ������������0.2mol A��0.2mol B��0.2mol C�����ʱv��������v���棩 |

 ���׳ơ����������������������谷���������ᣨ
���׳ơ����������������������谷���������ᣨ  �������������������谷�����֮��ͨ��
�������������������谷�����֮��ͨ��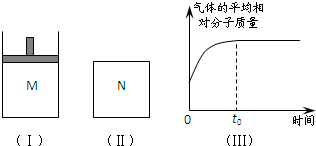 ��2008?����ģ�⣩��ͼ���ں�ѹ�ܱ�����M�м���2mol A��2mol B����ʼʱ�������ΪV L���������·�Ӧ���ﵽ��ѧƽ��״̬��2A������+B������?x C��g������H��0
��2008?����ģ�⣩��ͼ���ں�ѹ�ܱ�����M�м���2mol A��2mol B����ʼʱ�������ΪV L���������·�Ӧ���ﵽ��ѧƽ��״̬��2A������+B������?x C��g������H��0