��Ŀ����
(15�֣�ij��ѧС��Ϊ�ⶨ�ӵ�����KIO3�����Ƿ������Ʊ�����KIO3,����������ʵ�顣
I .�ⶨ�ӵ�����KIO3���������������ʲ�������Ӧ��
��֪��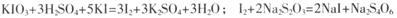
����һ��ȷ��ȡa g�ӵ��Σ����Ƴ�250 mL��Һ��
�������ȡ������Һ25.00 mL����ƿ�У���ϡ�����ữ���ټ�������KI��Һ��
���������Ե���Ϊָʾ������c mol.L-1��Na2S2O3��Һ�ζ������������Һ���յ㣬��¼���ݡ��ظ��ζ�2�Σ�ƽ������Na2S2Or��Һ12.00 mL��
(1) ����һ������250mL��Һ�õ��IJ����������ձ�������������Ͳ�⣬����____________
(2) �������е���ζ��յ�ʱ������Ϊ____________
(3) ʵ���ô˼ӵ�����KIO3����������="______" ______ (KIO3����Է�������Ϊ214)��
II.ʵ�����Ʊ�KIO3
��֪�������ο����������ڼ�����Һ�������⻯��õ���
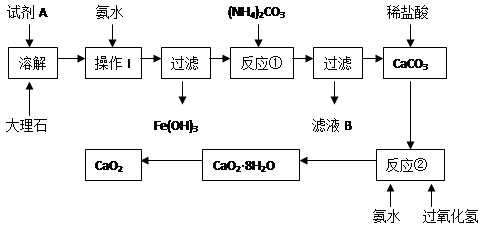
�û�ѧС��ͨ������װ���Ʊ�KIO3
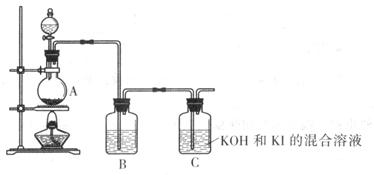
(4) װ��A�з�Ӧ�����ӷ���ʽΪ____________
(5) װ��B�е��Լ�Ϊ____________
(6) д��װ��C������KIO3�����ӷ���ʽ____________��
(7) ����ʵ��װ�ô���һ������ȱ�ݣ���ָ��: __________________��
I .�ⶨ�ӵ�����KIO3���������������ʲ�������Ӧ��
��֪��
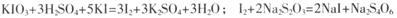
����һ��ȷ��ȡa g�ӵ��Σ����Ƴ�250 mL��Һ��
�������ȡ������Һ25.00 mL����ƿ�У���ϡ�����ữ���ټ�������KI��Һ��
���������Ե���Ϊָʾ������c mol.L-1��Na2S2O3��Һ�ζ������������Һ���յ㣬��¼���ݡ��ظ��ζ�2�Σ�ƽ������Na2S2Or��Һ12.00 mL��
(1) ����һ������250mL��Һ�õ��IJ����������ձ�������������Ͳ�⣬����____________
(2) �������е���ζ��յ�ʱ������Ϊ____________
(3) ʵ���ô˼ӵ�����KIO3����������="______" ______ (KIO3����Է�������Ϊ214)��
II.ʵ�����Ʊ�KIO3
��֪�������ο����������ڼ�����Һ�������⻯��õ���
�û�ѧС��ͨ������װ���Ʊ�KIO3

(4) װ��A�з�Ӧ�����ӷ���ʽΪ____________
(5) װ��B�е��Լ�Ϊ____________
(6) д��װ��C������KIO3�����ӷ���ʽ____________��
(7) ����ʵ��װ�ô���һ������ȱ�ݣ���ָ��: __________________��
��1��250mL����ƿ����ͷ�ιܣ�2�֣���1�֣���д250mL�ɲ��۷֣�
��2����Һ��ɫ��ɫ��������ڲ��ָ���ɫ��2�֣�
��3��(4.28c)/a��100%��3�֣������������������Ҳ�ɸ��֣�
��4��MnO2��4H����2Cl��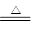 Mn2����Cl2����2H2O ��2�֣�
Mn2����Cl2����2H2O ��2�֣�
��5������ʳ��ˮ��2�֣�
��6��I����3Cl2��6OH����IO3����6 Cl����3H2O ��2�֣�
��7��ȱ�����պ���Cl2��β������װ�ã�����ɻ�����Ⱦ��2�֣�
��2����Һ��ɫ��ɫ��������ڲ��ָ���ɫ��2�֣�
��3��(4.28c)/a��100%��3�֣������������������Ҳ�ɸ��֣�
��4��MnO2��4H����2Cl��
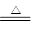 Mn2����Cl2����2H2O ��2�֣�
Mn2����Cl2����2H2O ��2�֣���5������ʳ��ˮ��2�֣�
��6��I����3Cl2��6OH����IO3����6 Cl����3H2O ��2�֣�
��7��ȱ�����պ���Cl2��β������װ�ã�����ɻ�����Ⱦ��2�֣�
��1������һ�����ʵ���Ũ����Һ�����ƣ�����ʵ��ԭ������֪��������֪����ȱ�ٵ���250mL����ƿ����ͷ�ιܡ�
��2�����ڵ��ۺ͵ⷴӦ����ɫ�������յ�ʱ��ʵ����������Һ��ɫ��ɫ��������ڲ��ָ���ɫ��
��3�����ù�ϵʽ���м��㡣���ݷ���ʽ��֪KIO3��3I2��6Na2S2O3������25ml��Һ�е���ص����ʵ�����0.012cmol��6��0.002cmol�����Դ˼ӵ�����KIO3������������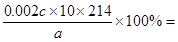
(4.28c)/a��100%��
��4��װ��A����ȡ�����ķ���װ�ã�����ʽΪMnO2��4H����2Cl��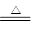 Mn2����Cl2����2H2O��
Mn2����Cl2����2H2O��
��5�����������ӷ���������ȡ�������к����Ȼ��⣬���Ȼ����ܺ��������Ʒ�Ӧ������ʵ��ʧ�ܣ�������ͨ��װ��C֮ǰ����Ҫ��ȥ�Ȼ��⣬�����ñ���ʳ��ˮ��ȥ�Ȼ��⡣
��6�������Ļ�ԭ�����������ӣ����Ը��ݷ�Ӧ����������֪������ʽΪI����3Cl2��6OH����IO3����6 Cl����3H2O��
��7�����������ж������Ա�����β������װ�ã���������ɻ�����Ⱦ��
��2�����ڵ��ۺ͵ⷴӦ����ɫ�������յ�ʱ��ʵ����������Һ��ɫ��ɫ��������ڲ��ָ���ɫ��
��3�����ù�ϵʽ���м��㡣���ݷ���ʽ��֪KIO3��3I2��6Na2S2O3������25ml��Һ�е���ص����ʵ�����0.012cmol��6��0.002cmol�����Դ˼ӵ�����KIO3������������
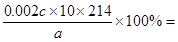
(4.28c)/a��100%��
��4��װ��A����ȡ�����ķ���װ�ã�����ʽΪMnO2��4H����2Cl��
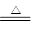 Mn2����Cl2����2H2O��
Mn2����Cl2����2H2O����5�����������ӷ���������ȡ�������к����Ȼ��⣬���Ȼ����ܺ��������Ʒ�Ӧ������ʵ��ʧ�ܣ�������ͨ��װ��C֮ǰ����Ҫ��ȥ�Ȼ��⣬�����ñ���ʳ��ˮ��ȥ�Ȼ��⡣
��6�������Ļ�ԭ�����������ӣ����Ը��ݷ�Ӧ����������֪������ʽΪI����3Cl2��6OH����IO3����6 Cl����3H2O��
��7�����������ж������Ա�����β������װ�ã���������ɻ�����Ⱦ��

��ϰ��ϵ�д�
�����Ŀ
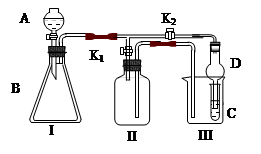
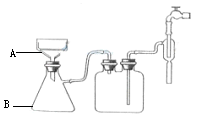
 ��
��
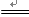 CaSO4 + H2O + CO2��
CaSO4 + H2O + CO2��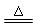 MnCl2 + Cl2��+ 2H2O
MnCl2 + Cl2��+ 2H2O