��Ŀ����
����Ŀ������98%��Ũ����(p��1.84g��cm-3 )���Ƴ�Ũ��Ϊ0.5mol��L-1��ϡ����500ml��
(1)����ʱ����IJ��������У�________________��
(2)�����ù����У����в�������ȷ����(�����)__________��
A��ʹ������ƿǰ������Ƿ�©ˮ
B������ƿ������ˮϴ�������ô���Һ��ϴ
C�����������ƹ��������ƽ���̵���ֽ�ϣ�ȷ�����������ձ����ܽ�.
D����ȷ��ȡ��Ũ���ᣬע����ʢ��30mLˮ��100mL������ƿ�У���ˮ���̶���
E�����ݺ�����ƿ������ʳָ��סƿ��������һֻ�ֵ���ָ��סƿ�ף�������ƿ��תҡ��
(3)��Ҫ�ش��������⣺
������Ũ��������Ϊ____________mL��
�����ʵ������15mL��20mL��50mL����ͲӦѡ��____________mL����Ͳ���.
(4)�����ƹ����У���ijѧ���۲춨��ʱ����Һ�棬������Һ��Ũ�Ȼ�________(����ƫ��������ƫ����������Ӱ������ͬ)����δ����ȴ���Ƚ���Һע������ƿ�У�_______��������ƿ��ԭ����������ˮ��________��
���𰸡���Ͳ�����������ձ�����ͷ�ιܡ�500mL����ƿ B C D 13.6 15 ƫ�� ƫ�� ��Ӱ��
��������
��1���������Ʋ����Ǽ��㡢��ȡ��ϡ�͡���Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��������Ҫ��������
��2���������Ʋ���Ҫ���ʵ��淶�жϣ�
��3�����ȼ����Ũ��������ʵ���Ũ��Ϊc=![]() ��Ȼ�������Һϡ�Ͷ���CŨVŨ=CϡVϡ�����㣻
��Ȼ�������Һϡ�Ͷ���CŨVŨ=CϡVϡ�����㣻
�ڸ��������������ԭ������Ҫ��ȡ��Ũ����������ѡ����ʵ���Ͳ��
��4������c=![]() ��������ʵ����ʵ���n����Һ�����V�ı仯��������������
��������ʵ����ʵ���n����Һ�����V�ı仯��������������
��1�����������м��㡢��ȡ��ϡ�͡�ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ������Ͳ��ȡŨ���ᣬ���ձ���ϡ�ͣ�������Ͳ��ȡˮ�����ձ��������ò��������裮��ȴ��ת�Ƶ�500mL����ƿ�У����ò�����������ϴ���ձ���������2-3�Σ�����ϴ��Һ��������ƿ�У���ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ�����������������Ͳ���ձ�����������500mL����ƿ����ͷ�ιܣ�
�ʴ�Ϊ����Ͳ���ձ�����������500mL����ƿ����ͷ�ιܣ�
��2��A������ƿ�Ǵ������ӵ�������ʹ������ƿǰ������Ƿ�©ˮ����A��ȷ��
B������ƿ������ˮϴ���������ô���Һ��ϴ������������ҺŨ��ƫ��B����
C���������ƾ�����ˮ�ԣ����ܷ�����ֽ�ϳ�����Ҫ���������ƹ�������ձ���������C����
D������ƿ�����ڹ涨���¶���ʹ�ã���������ϡ��Ũ���ᣬ���������Ϲ淶����D����
E��ҡ�ȵIJ��������ݺ�����ƿ������ʳָ��סƿ��������һֻ�ֵ���ָ��סƿ�ף�������ƿ��תҡ�ȣ���E��ȷ��
�ʴ�Ϊ��B C D��
��3����Ũ��������ʵ���Ũ��Ϊc=![]() =18.4mol��L��1������Ҫ��Ũ��������ΪVml��������Һϡ�Ͷ���CŨVŨ=CϡVϡ��֪��18.4mol��L��1��VmL=0.5mol��L��1��500mL�����V=13.6mL���ʴ�Ϊ��13.6��
=18.4mol��L��1������Ҫ��Ũ��������ΪVml��������Һϡ�Ͷ���CŨVŨ=CϡVϡ��֪��18.4mol��L��1��VmL=0.5mol��L��1��500mL�����V=13.6mL���ʴ�Ϊ��13.6��
����Ҫ��ȡ��Ũ��������Ϊ13.6mL��������Ͳ��ѡ���������������ԭ��ѡ��15mL����Ͳ���ʴ�Ϊ��15��
��4���ٶ���ʱ����Һ�棬������Һ�������������Һ��Ũ�Ȼ�ƫ�ͣ���δ����ȴ���Ƚ���Һע������ƿ�У���ȴ��������Һ�����С��������Һ��Ũ�Ȼ�ƫ�ߣ��۶���ʱ����Ҫ�������������ˮ��������ƿ��ԭ����������ˮ����������Һ��Ӱ�죻�ʴ�Ϊ��ƫ�ͣ�ƫ�ߣ���Ӱ�졣

����Ŀ������ʵ��װ�ò��ܴﵽʵ��Ŀ�ĵ���
A | B | C | D |
|
|
|
|
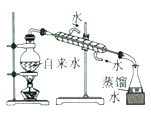
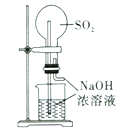
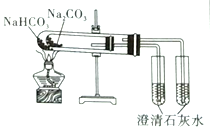
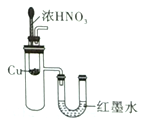
ʵ������ȡ����ˮ | ��SO2��NaOH��Һ����Ȫʵ�� | ֤��Na2CO3�����ȶ��Ա�NaHCO3�� | ֤��ͭ��Ũ����ķ�Ӧ�Ƿ��ȷ�Ӧ |
A. A B. B C. C D. D