��Ŀ����
��6�֣�����������N2H4Ϊȼ��,NO2Ϊ������,���߷�Ӧ����N2��ˮ����, ��֪
N2(g)+2O2(g)=2NO2(g) �S H1 �� �� 67.7kJ/mol��
N2H4(g)+O2(g)= N2(g)+2H2O(g) �S H2 �� �� 534kJ/mol��
2H2(g)+ O2(g)= 2H2O(g) �S H3 �� �� 484kJ/mol��
H2(g)+F2(g)=2HF(g) �S H4 �� �� 538kJ/mol
д��N2H4�� NO2��Ӧ���Ȼ�ѧ����ʽ________________________________________,
д��N2H4��F2��Ӧ���Ȼ�ѧ����ʽ__________________________________________��
N2(g)+2O2(g)=2NO2(g) �S H1 �� �� 67.7kJ/mol��
N2H4(g)+O2(g)= N2(g)+2H2O(g) �S H2 �� �� 534kJ/mol��
2H2(g)+ O2(g)= 2H2O(g) �S H3 �� �� 484kJ/mol��
H2(g)+F2(g)=2HF(g) �S H4 �� �� 538kJ/mol
д��N2H4�� NO2��Ӧ���Ȼ�ѧ����ʽ________________________________________,
д��N2H4��F2��Ӧ���Ȼ�ѧ����ʽ__________________________________________��
��1��2N2H4(g)+2NO2(g)=3N2(g)+4H2O(g) ��H���C1135.7 kJ��mol��1��
��2��N2H4(g)+2F2(g)=N2(g)+4HF(g) ��H���C1126kJ��mol��1��
��2��N2H4(g)+2F2(g)=N2(g)+4HF(g) ��H���C1126kJ��mol��1��
���������������1�� N2(g)+2O2(g)=2NO2(g) �S H1 �� �� 67.7kJ/mol��
��2�� N2H4(g)+O2(g)= N2(g)+2H2O(g) �S H2 �� �� 534kJ/mol��
��3�� 2H2(g)+ O2(g)= 2H2O(g) �S H3 �� �� 484kJ/mol��
��4�� H2(g)+F2(g)=2HF(g) �S H4 �� �� 538kJ/mol
�ѣ�2��´2-��1�������ø�˹���ɿɵã�
2N2H4(g)+2NO2(g)=3N2(g)+4H2O(g) ��H=�� 534kJ/mol´2-67.7kJ/mol= �C1135.7kJ��mol��1
�ѣ�2��-��3��-��4��´2�����ø�˹���ɿɵã�
N2H4(g)+2F2(g)=N2(g)+4HF(g)
��H���� 534kJ/mol-���� 484kJ/mol��-���� 538kJ/mol��´2= �C1126kJ��mol��1
���������⿼���ø�˹���ɽ����йط�Ӧ�ȵļ��㡣Ҫ��ѧ���Ը�˹�������սϺá�

��ϰ��ϵ�д�
�����Ŀ

 ��
�� 

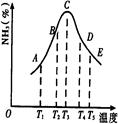
 2NH3�����ֱ���t��ʱ�ⶨ����NH3�������������ͼ���ң�
2NH3�����ֱ���t��ʱ�ⶨ����NH3�������������ͼ���ң�