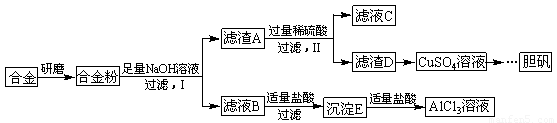
��Ŀ����
ijѧУ��ѧ��ȤС��Ϊ̽��������������ۺ����ã�ר�����ʵ���ú�����������ͭ�ĺϽ���ȡ�������Ȼ�����Һ���̷�����(![]() )�͵�������(
)�͵�������(![]() )����ʵ�鷽�����£�
)����ʵ�鷽�����£�

�Իش��������⣺
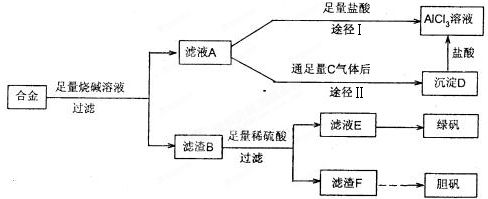
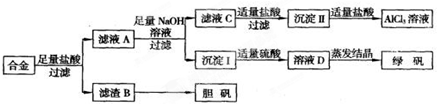
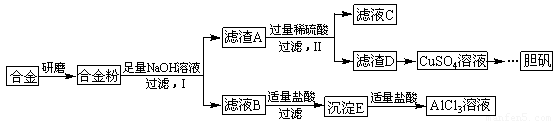
(1)�����õIJ���������_______________________��
(2)д���ɳ�����ת��ΪAlC13��Һ�����ӷ���ʽ_____________________��
(3)С���Ա������⣮�����Ƶõ��̷��������Է���ԭ��___________________��Ҫ���ɳ���I�����Ƶô��Ƚϸߵ��̷���Ӧ��θĽ�_________________________
(4)С���Ա�������л�֪H2O����һ����ɫ��������������B�м���ϡ�����H2O�����Ʊ��������壬��÷�Ӧ���ܻ�ѧ����ʽΪ________________________________
(5)��ͬѧ����ɽ�����������ܽ�Ͻ����������ռ������Ʒ�����Ҳ�������Ƶ��������ʣ�����Ϊ���߷��������ǰ����______(�����������������)��������_____
��1���ձ���©����������
��2��![]()
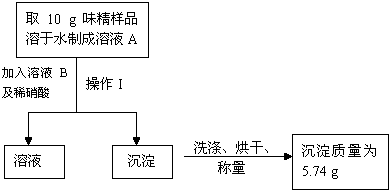
��3����Ӧ�����в��ֶ�����ת������������������ҺD�м����������ۣ���Ӧ����ˣ�ȡ��Һ�����ᾧ���ɵ��̷�
��4��![]()
��![]()
��5����������2�֣���Ϊǰһ�ַ�����������ࡢʱ�䳤�������Լ�������