��Ŀ����
20��ij��ѧ��ȤС������ͼ1��ʾװ�ý���̽��ʵ�飬����֤����������ϩ��������ϩ���в������ԣ����¶�Ѹ�������ɹ۲쵽�Թ�����ˮ��ɫ����ƿ��ŨH2SO4���Ҵ��Ļ��Һ���Ϊ�غ�ɫ��?
��1��д����ʵ����������ϩ�Ļ�ѧ����ʽCH3CH2OH$��_{170��}^{Ũ����}$H2O+CH2=CH2����
��2����ͬѧ��Ϊ�����ǵ��û��Һ�巴Ӧ�ĸ����ԣ���ˮ��ɫ��������֤����Ӧ������ϩ��������ϩ���в������ԣ���������ȷ����bc��
a����ϩ����ˮ����ȡ����Ӧ?b��ʹ��ˮ��ɫ�ķ�Ӧ��δ���Ǽӳɷ�Ӧ?
c��ʹ��ˮ��ɫ�����ʣ�δ������ϩ?
��3����ͬѧ����ϸ�¹۲���Թ�����һʵ�������֤����Ӧ������ϩ���ɣ������������һʵ������۲쵽Һ��ֲ㣬�²�Ϊ��״Һ�壮
��4����ͬѧ������ʵ��װ�ý����˸Ľ����ڢ�͢�֮������ͼ2װ���Գ�ȥ�Ҵ�������SO2����A�е��Լ���NaOH��Һ��B�е��Լ�ΪƷ����Һ��
���� ��1����Ũ������������170����������£��Ҵ�������ȥ��Ӧ������ϩ��
��2��Ũ�������ǿ�����ԣ��ܽ��Ҵ���������̼����������ԭ���ɶ��������������л�ԭ�ԣ�
��3����ϩ���巢���ӳɷ�Ӧ����������������������ˮ���ܶȴ���ˮ��
��4�����������������������NaOH��Һ�ܽ������������գ�����������ʹƷ����Һ��ɫ��
��� �⣺��1����Ũ������������170����������£��Ҵ�������ȥ��Ӧ������ϩ����Ӧ����ʽΪ��CH3CH2OH$��_{170��}^{Ũ����}$H2O+CH2=CH2����
�ʴ�Ϊ��CH3CH2OH$��_{170��}^{Ũ����}$H2O+CH2=CH2����
��2��ŨH2SO4����ǿ�����ԣ����Ҵ�������̼����������ԭ���ɶ�������C+2H2SO4 $\frac{\underline{\;\;��\;\;}}{\;}$CO2��+2SO2��+2H2O��SO2���л�ԭ�ԣ�������Br2����������ԭ��Ӧ����������������ʹ����ɫ����Ӧ����ʽΪSO2+Br2+2H2O=2HBr+H2SO4������bc��ȷ��
��ѡ��bc��
��3����ˮ�е����뷴Ӧ���ɵ���ϩ�����ӳɷ�Ӧ������ʽΪ��CH2=CH2+Br2��CH2BrCH2Br��1��2-���������ܶȴ���ˮ���Թܵײ�Ϊ��״���ʣ�
�ʴ�Ϊ���۲쵽Һ��ֲ㣬�²�Ϊ��״Һ�壻
��4�����������ܺ��巢��������ԭ��Ӧ��ʹ��ˮ��ɫ���Ӷ�������ϩ�ļ��飬����������������������ܺͼӦ�����Σ�Ϊ��ֹ�������������ϩ�ļ��飬����������������Һ��ȥ����������������ʹƷ����Һ��ɫ�����Կ�����Ʒ����Һ�����������ʵ��ʱҪ�����ն��������ټ�����������Ƿ�������A�е��Լ���NaOH��Һ��B��Һ����Ʒ����Һ��
�ʴ�Ϊ��NaOH��Һ��Ʒ����Һ��
���� ���⿼����ʵ������ȡ��ϩʵ�飬��ȷʵ��ԭ���ǽⱾ��ؼ�������Ũ���ᡢ��ϩ���������������ע�����������ϩ����ʹ��ˮ��ɫ������ɫԭ����ͬ����ϩʹ��ˮ��ɫ�Ƿ����˼ӳɷ�Ӧ����������ʹ��ˮ��ɫ�Ƿ�����������ԭ��Ӧ��

| A�� | ��״����22.4LNO2�з�����ԼΪNA�� | |
| B�� | �����£�18g${\;}_{\;}^{18}$O2�к��и�NA��ԭ�� | |
| C�� | 31g�����к��Ĺ��ۼ���Ϊ3NA | |
| D�� | 25��ʱpH=13��NaOH��Һ�к���0.1NAOH- |
| A�� | 8��11 | B�� | 8��13 | C�� | 6��8 | D�� | 12��9 |
| A�� | ԭ�������ʵ���Ũ�ȵ���0.01mol•L-1 | |
| B�� | ��Ӧ�����Һ�У�c��CH3COO-��+c��CH3COOH��=0.01mol•L-1 | |
| C�� | ���ַ�Ӧ����ˮ�������c��H+������1��10-12mol•L-1 | |
| D�� | ��Ӧ�����Һ�У�c��CH3COO-����c��Na+����c��OH-����c��H+�� |
| A�� | ��˾ƥ�� | B�� | ��Ī���� | C�� | ��ð�� | D�� | θ���� |
 B
B E
E
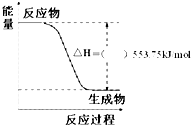 ��1���������˻����ƻ�潫�й���ͳ�Ļ������˾����Լ��ִ��߿Ƽ���Ϊһ�壮��������ܴ����Դ�ڱ����ȼ�գ�������һ��������ȼ�ϣ��Իش��������⣺
��1���������˻����ƻ�潫�й���ͳ�Ļ������˾����Լ��ִ��߿Ƽ���Ϊһ�壮��������ܴ����Դ�ڱ����ȼ�գ�������һ��������ȼ�ϣ��Իش��������⣺