��Ŀ����
3���������С�ظ���Ԫ��Ҳ�ƾ����������������壬�����������8���������һ����ԭ�ӣ��������������һ����ԭ�ӣ�ÿ����ԭ�ӱ����ڵľ��������ã���ͼ�����ٶ���ԭ���Ǹ���С��ÿ��ԭ�ӽ��ܶѻ�����ԭ�ӵ�ֱ��Ϊd cm����NA��ʾ�����ӵ�������M��ʾ���Ħ��������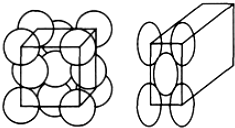
��1������ÿ�������к���4����ԭ�ӣ�
��2��һ�������������2$\sqrt{2}$d3cm3
��3��������ܶ���$\frac{2M}{\sqrt{2}{d}^{3}{N}_{A}}$g/cm3��
���� ��1����ԭ��λ�����ĺͶ��㣬���ݾ�̯���������ÿ�������н�ԭ������
��2�����ݾ����ı߳������С��Ĺ�ϵ��ñ߳������ɱ߳����㾧���������
��3����֪ÿ����������4����ԭ�ӣ�����������������ٸ��ݦ�=$\frac{m}{V}$�����ܶȣ�
��� �⣺��1����ԭ��λ�����ĺͶ��㣬���ݾ�̯���ɼ������ÿ�������н�ԭ����Ϊ$\frac{1}{8}$��8+$\frac{1}{2}$��6=4��
�ʴ�Ϊ��4��
��2�����ݾ����Ľṹͼ��֪�������ĶԽ��߳�Ϊ2dcm�����Ծ����ı߳�Ϊ$\frac{\sqrt{2}}{2}$��2d=$\sqrt{2}$dcm�����Ծ��������Ϊ$��\sqrt{2}d��^{3}$=2$\sqrt{2}$d3cm3��
�ʴ�Ϊ��2$\sqrt{2}$d3��
��3����֪ÿ����������4����ԭ�ӣ���������Ϊm=$\frac{4M}{{N}_{A}}$g�����Ԧ�=$\frac{m}{V}$=$\frac{\frac{4M}{{N}_{A}}}{2\sqrt{2}{d}^{3}}$=$\frac{2M}{\sqrt{2}{d}^{3}{N}_{A}}$g/cm3��
�ʴ�Ϊ��$\frac{2M}{\sqrt{2}{d}^{3}{N}_{A}}$��
���� ������Ҫ�����˾����ļ��㣬�漰��̯�����ܶȵļ��㣬�Ѷ��еȣ�����ʱҪע�������йؼ���ķ�����

| A�� | ���ռ���Һ����NO2��2NO2+2OH-=NO3-+NO2-+H2O | |
| B�� | ���ռ���Һ����������Cl2+2OH-=Cl-+ClO-+H2O | |
| C�� | ������Һ�������ˮ��Ӧ��Al3++3NH3��H2O=Al��OH��3��+3NH4+ | |
| D�� | ����KI������Һ���ú������4I-+O2+2H2O=2I2+4OH- |
�Լ�������ˮ ��Ũ���� �۵�ˮ ������������ͭ��
����A����ɫ��ȥ B������ɫ C�����ֺ�ɫ���� D���ʻ�ɫ
| �л��� | �Լ� | ���� |
| ��1����ϩ | ||
| ��2�������� | ||
| ��3������ | ||
| ��4�������� |
| X | Y | |||
| W | Z |
| A�� | ����������ˮ��������ԣ�X��Z | |
| B�� | ԭ�Ӱ뾶��С��Y��W | |
| C�� | ��ҵ���õ�����ڵ�W��Z���γɵĻ���������ȡW | |
| D�� | W��Y�γɵĻ��������Z���⻯���ˮ��Һ������Ӧ |
| A�� | NaCl SiO2 CO2 | B�� | NaCl CO2 SiO2 | ||
| C�� | CO2 NaCl SiO2 | D�� | SiO2 NaCl CO2 |
| A�� |  | B�� |  | C�� |  | D�� |  |
| A�� | �ﵽ��ѧƽ��ʱ��N2��ȫת��ΪNH3 | |
| B�� | һ�������´ﵽ��ѧƽ��ʱ��N2��H2��NH3�����ʵ���Ũ��֮��Ϊ1��3��2 | |
| C�� | ��λʱ��������a mol N2ͬʱ����2amol NH3��˵���÷�Ӧ�Ѵﵽƽ��״̬ | |
| D�� | �ﵽ��ѧƽ��ʱ������Ӧ���淴Ӧ������ȣ��Ҷ�Ϊ�� |
| ����� | �Լ� | ���뷽�� | |
| A | �������ӣ� | Ũ��ˮ | ���� |
| B | ��ˮ��ˮ�� | ������ | ��ȡ |
| C | �������������ᣩ | ����Na2CO3��Һ | ��Һ |
| D | ��������Һ���Ȼ�����Һ�� | ����ˮ | ���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ���ӣ�C6H5OH���ܸ�NaOH��Һ��Ӧ���Ҵ����� | |
| B�� | ��ϩ�ܷ����ӳɷ�Ӧ�����鲻�� | |
| C�� | �ױ���ʹKMnO4������Һ��ɫ�����鲻�� | |
| D�� | ����50�桫60��ʱ����������Ӧ���ױ���30��ʱ���� |