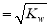МвДҝДЪИЭ
ДіКөСйРЎЧйЙијЖУГ50 mL 1.0 mol/LСОЛбёъ50 mL 1.1 mol/L ЗвСх»ҜДЖИЬТәФЪИзНјЧ°ЦГЦРҪшРРЦРәН·ҙУҰЎЈФЪҙуЙХұӯөЧІҝөжЛйЕЭДӯЛЬБП(»тЦҪМх)Ј¬К№·ЕИлөДРЎЙХұӯұӯҝЪУлҙуЙХұӯұӯҝЪПаЖҪЎЈИ»әуФЩФЪҙуЎўРЎЙХұӯЦ®јдМоВъЛйЕЭДӯЛЬБП(»тЦҪМх)Ј¬ҙуЙХұӯЙПУГЕЭДӯЛЬБП°е(»тУІЦҪ°е)ЧчёЗ°еЈ¬ФЪ°еЦРјдҝӘБҪёцРЎҝЧЈ¬ХэәГК№ОВ¶ИјЖәН»·РОІЈБ§ҪБ°и°фНЁ№эЎЈНЁ№эІв¶Ё·ҙУҰ№эіМЦРЛщ·ЕіцөДИИБҝҝЙјЖЛгЦРәНИИЎЈКФ»ШҙрПВБРОКМвЈә
ЈЁ1Ј©ұҫКөСйЦРУГЙФ№эБҝөДNaOHөДФӯТтҪМІДЦРЛөКЗОӘұЈЦӨСОЛбНкИ«ұ»ЦРәНЎЈКФОКЈәСОЛбФЪ·ҙУҰЦРИфТтОӘУР·ЕИИПЦП󣬶шФміЙЙЩБҝСОЛбФЪ·ҙУҰЦР»У·ўЈ¬ФтІвөГөДЦРәНИИ (МоЎ°Ж«ҙуЎұЎўЎ°Ж«РЎЎұ»тЎ°І»ұдЎұ)ЎЈ
ЈЁ2Ј©ФЪЦРәНИИІв¶ЁКөСйЦРҙжФЪУГЛ®ПҙөУОВ¶ИјЖЙПөДСОЛбөДІҪЦиЈ¬ИфОЮҙЛІЩЧчІҪЦиЈ¬ФтІвөГөДЦРәНИИ»б (МоЎ°Ж«ҙуЎұЎўЎ°Ж«РЎЎұ»тЎ°І»ұдЎұ)ЎЈ
ЈЁ3Ј©ИфУГөИЕЁ¶ИөДҙЧЛбУлNaOHИЬТә·ҙУҰЈ¬ФтІвөГөДЦРәНИИ»б (МоЎ°Ж«ҙуЎұЎўЎ°Ж«РЎЎұ»тЎ°І»ұдЎұ)Ј¬ЖдФӯТтКЗ ЎЈ
ЈЁ4Ј©ёГКөСйРЎЧйЧцБЛИэҙОКөСйЈ¬ГҝҙОИЎИЬТәёч50 mLЈ¬ІўјЗВјПВФӯКјКэҫЭ(јыПВұн)ЎЈ
КөСйРтәЕ | ЖрКјОВ¶Иt1/Ўж | ЦХЦ№ОВ¶И(t2)/Ўж | ОВІо(t2Јӯt1)/Ўж | ||
СОЛб | NaOHИЬТә | ЖҪҫщЦө | |||
1 | 25.1 | 24.9 | 25.0 | 31.6 | 6.6 |
2 | 25.1 | 25.1 | 25.1 | 31.8 | 6.7 |
3 | 25.1 | 25.1 | 25.1 | 31.9 | 6.8 |
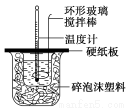
ТСЦӘСОЛбЎўNaOHИЬТәГЬ¶ИҪьЛЖОӘ1.00g/cm3Ј¬ЦРәНәу»мәПТәөДұИИИИЭcЈҪ4.18ЎБ10Јӯ3kJ/(gЎӨЎж)Ј¬ФтёГ·ҙУҰөДЦРәНИИОӘҰӨHЈҪ ЎЈёщҫЭјЖЛгҪб№ыЈ¬РҙіцёГЦРәН·ҙУҰөДИИ»ҜС§·ҪіМКҪ ЎЈ
(1)Ж«РЎ (2) Ж«РЎЈЁ3Ј©Ж«РЎЈЁ4Ј© Јӯ56.01 kJ/mol
ЎҫҪвОцЎҝ
КФМв·ЦОцЈәЈЁ1Ј©ИфТтОӘУР·ЕИИПЦПуөјЦВЙЩБҝСОЛбФЪ·ҙУҰЦР»У·ўЈ¬јхЙЩБЛHClөДБҝЈ¬өјЦВЙъіЙөДЛ®өДОпЦКөДБҝЖ«РЎЈ¬№КІвөГөДЦРәНИИ»бЖ«РЎЈ¬
ЈЁ2Ј©Г»УРУГЛ®ПҙөУОВ¶ИјЖЙПөДСОЛбИЬТәЈ¬өјЦВСОЛбөДОпЦКөДБҝЖ«РЎЈ¬·ЕіцөДИИБҝЖ«РЎЈ¬ІвөГөДЦРәНИИКэЦөЖ«РЎЈ¬
ЈЁ3Ј©УЙУЪҙЧЛбОӘИхЛбЈ¬ҙЧЛбөзАлТӘОьКХДЬБҝЈ¬ФміЙІвөГөДЦРәНИИЖ«РЎЈ¬
№Кҙр°ёОӘЈәЖ«РЎЈ»УГҙЧЛбҙъМжСОЛбЈ¬ҙЧЛбөзАлТӘОьКХДЬБҝЈ¬ФміЙІвөГөДЦРәНИИЖ«РЎЈ»
ЈЁ4Ј©ұнЦРИэҙОІвБҝКэҫЭ¶јКЗУРР§өДЈ¬ИэҙООВІоөДЖҪҫщЦөОӘЈә
ҰӨHЈҪЈӯ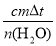 ЈҪЈӯ
ЈҪЈӯ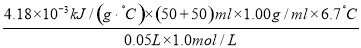 ЈҪЈӯ56.01 kJ/molЎЈ
ЈҪЈӯ56.01 kJ/molЎЈ
ёщҫЭЦРәНИИөДёЕДоҝЙЦӘЈ¬ИИ»ҜС§·ҪіМКҪОӘЈәHClЈЁaqЈ©+NaOHЈЁaqЈ©=NaClЈЁaqЈ©+H2OЈЁlЈ©ЎчH=-57.3KJ/molЈ»
№Кҙр°ёОӘЈә-56.01 kJ/molЈ»HClЈЁaqЈ©+NaOHЈЁaqЈ©=NaClЈЁaqЈ©+H2OЈЁlЈ©ЎчH=-57.3KJ/molЈ®
ҝјөгЈәұҫМвҝјІйБЛЦРәНИИөДіБөн

 МмМмПтЙПТ»ұҫәГҫнПөБРҙр°ё
МмМмПтЙПТ»ұҫәГҫнПөБРҙр°ё РЎС§Йъ10·ЦЦУУҰУГМвПөБРҙр°ё
РЎС§Йъ10·ЦЦУУҰУГМвПөБРҙр°ёёЯВИЛбЎўБтЛбЎўПхЛбәНСОЛб¶јКЗЗҝЛбЈ¬ЖдЛбРФФЪЛ®ИЬТәЦРІоұрІ»ҙуЎЈТФПВКЗДіОВ¶ИПВХвЛДЦЦЛбФЪұщҙЧЛбЦРөДөзАліЈКэЈәҙУПВұнёсЦРЕР¶ППВБРЛө·ЁХэИ·өДКЗ
Лб | HClO4 | H2SO4 | HCl | HNO3 |
Ka | 1.6ЎБ10-5 | 6.3ЎБ10-9 | 1.6ЎБ10-9 | 4.2ЎБ10-10 |
AЈ®ФЪұщҙЧЛбәНЛ®ЦРХвЛДЦЦЛб¶јГ»УРНкИ«өзАл
BЈ®ФЪұщҙЧЛбЦРёЯВИЛбКЗХвЛДЦЦЛбЦРЧоИхөДЛб
CЈ®ФЪұщҙЧЛбЦРБтЛбөДөзАл·ҪіМКҪОӘH2SO4=2H+Ј«SO42-
DЈ®Л®¶ФУЪХвЛДЦЦЛбөДЗҝИхГ»УРЗш·ЦДЬБҰЈ¬ө«ҙЧЛбҝЙТФЗшұрХвЛДЦЦЛбөДЗҝИх
ҪсУРКТОВПВЛДЦЦИЬТәЈ¬УР№ШРрКцІ»ХэИ·өДКЗ
| ўЩ | ўЪ | ўЫ | ўЬ |
pH | 11 | 11 | 3 | 3 |
ИЬТә | °ұЛ® | ЗвСх»ҜДЖИЬТә | ҙЧЛб | СОЛб |
AЈ®ТСЦӘҙЧЛбп§ИЬТәіКЦРРФЈ¬ФЪўЩЎўўЪБҪЦР·ЦұрјУИлККБҝөДВИ»Ҝп§ҫ§Ме»тҙЧЛбп§ҫ§МеәуЈ¬БҪИЬТәөДpHҫщјхРЎ
BЈ®ЛДЦЦИЬТә·ЦұрјУЛ®ПЎКН10ұ¶Ј¬ЛДЦЦИЬТәөДpH ўЩЈҫўЪЈҫўЬЈҫўЫ
CЈ®ўЩЎўўЬБҪИЬТәөИМе»э»мәПЈ¬ЛщөГИЬТәЦРc(ClЈӯ)Јҫc(NH4+)Јҫc(H+)Јҫc(OHЈӯ)
DЈ®VaLўЬИЬТәУлVbLўЪИЬТә»мәПәу,Иф»мәПәуИЬТәpHЈҪ4, ФтVa ЎГVbЈҪ11ЎГ9
ДіН¬С§УГ№ӨТөБтЛбНӯЈЁә¬БтЛбСЗМъөИФУЦКЈ©ЦЖұёҙҝҫ»өДCuSO4ЎӨ5H2OЎЈ№ӨТХБчіМИзПВ
ЈЁІҝ·ЦІЩЧчәНМхјюВФЈ©Јә
IЈ®ИЎ№ӨТөБтЛбНӯ№ММеЈ¬УГПЎБтЛбИЬҪвЈ¬№эВЛЎЈ
IIЈ®ПтВЛТәЦРөОјУH2O2ИЬТәЈ¬ЙФјУИИЎЈ
IIIЈ®ПтIIөДИЬТәЦРјУИлCuO·ЫД©ЦБpHЈҪ4ЎЈ
IVЈ®јУИИЦу·РЈ¬№эВЛЈ¬ВЛТәУГПЎБтЛбЛб»ҜЦБpHЈҪ1ЎЈ
VЈ®Хф·ўЕЁЛхЎўАдИҙҪбҫ§Ўў№эВЛЎўПҙөУЎўёЙФпЈ¬өГҫ§МеЎЈ
ТСЦӘІҝ·ЦСфАлЧУЙъіЙЗвСх»ҜОпөДpHЎўKspЈЁ25ЎжЈ©ИзПВұнЈә
ОпЦК | Fe(OH)3 | Fe (OH)2 | Cu(OH)2 |
ҝӘКјіБөнКұpH | 2.7 | 7.6 | 4.7 |
НкИ«іБөнКұpH | 3.7 | 9.6 | 6.7 |
Ksp | 4.0ЎБ10ЁC38 | 8.0ЎБ10ЁC16 | 2.2ЎБ10ЁC20 |
ЈЁ1Ј©IIЦР·ўЙъ·ҙУҰөДАлЧУ·ҪіМКҪКЗ ЎЈ
ЈЁ2Ј©IIЦРҪ«Fe2+Сх»ҜОӘFe3+өДДҝөДКЗ ЎЈ
ЈЁ3Ј©УГK3[Fe(CN)6]ЈЁМъЗи»ҜјШЈ©СйЦӨIIЦРFe2+КЗ·сЧӘ»ҜНкИ«өДПЦПуКЗ ЎЈ
ЈЁ4Ј©IIIЦР·ўЙъ·ҙУҰөДАлЧУ·ҪіМКҪКЗ ЎЈ
НЁ№эјЖЛгЛөГчФЪҙЛМхјюПВөДИЬТәЦРFe3+КЗ·сіБөнНкИ«________________________(МбКҫЈәөұИЬТәЦРДіАлЧУЕЁ¶ИРЎУЪ1.0ЎБ10ЁC5 mol/LКұҝЙИПОӘёГАлЧУіБөнНкИ«)ЎЈ
ЈЁ5Ј©УҰУГ»ҜС§ЖҪәвТЖ¶ҜФӯАнҪвКНIVЦРЎ°ВЛТәУГПЎБтЛбЛб»ҜЎұөДФӯТт ЎЈ