��Ŀ����
8��A��B��C��D��E��F��G��H��I��J��Ϊ�л�����������ͼ�ش���������
��1��B��C��Ϊ��֧�����л������B�Ľṹ��ʽΪ��CH3��2CHCOOH��C��Ũ���������¼��ȷ�Ӧֻ������һ��ϩ��D��D�Ľṹ��ʽΪ��CH3��2C�TCH2��
��2��G�ܷ���������Ӧ��Ҳ��ʹ������Ȼ�̼��Һ��ɫ����G�Ľṹ��ʽΪ��CH2=C��CH3��-CHO��
��3���ݵķ�Ӧ������ˮ�ⷴӦ����ķ�Ӧ�����ǼӾ۷�Ӧ��
��4���ٷ�Ӧ���ͣ�ˮ�ⷴӦ�ܷ�Ӧ���ͣ��ӳɷ�Ӧ��������Ӧ
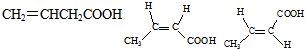
��5����H������ͬ�����ŵ�H��ͬ���칹��Ľṹ��ʽ��
 ��
��
���� ����A��B+C��ˮ�ⷴӦ�������ж�A��B��C�ֱ�����������ʹ������ɣ�1������ȷ��B��C�Ľṹ�ֱ�Ϊ��CH3��2CHCOOH�ͣ�CH3��2CHCH2OH��C��Ũ���������¼��ȷ�Ӧֻ������һ��ϩ��D����DΪ��CH3��2C=CH2����D��E��ȡ����Ӧ��EΪ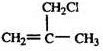 ��E����ˮ��õ���F��GΪȩ�Һ���˫��������д����ṹΪCH2=C��CH3��-CHO��������Ӧ�ߵõ�����HΪCH2=C��CH3��-COOH��H��CH3OH�õ���IΪCH2=C��CH3��-COOCH3����JΪ�Ӿ۷�Ӧ�IJ��Ϊ
��E����ˮ��õ���F��GΪȩ�Һ���˫��������д����ṹΪCH2=C��CH3��-CHO��������Ӧ�ߵõ�����HΪCH2=C��CH3��-COOH��H��CH3OH�õ���IΪCH2=C��CH3��-COOCH3����JΪ�Ӿ۷�Ӧ�IJ��Ϊ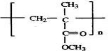 ������л���Ľṹ�������Լ���ĿҪ��ɽ����⣮
������л���Ľṹ�������Լ���ĿҪ��ɽ����⣮
��� �⣺����A��B+C��ˮ�ⷴӦ�������ж�A��B��C�ֱ�����������ʹ������ɣ�1������ȷ��B��C�Ľṹ�ֱ�Ϊ��CH3��2CHCOOH�ͣ�CH3��2CHCH2OH��C��Ũ���������¼��ȷ�Ӧֻ������һ��ϩ��D����DΪ��CH3��2C=CH2����D��E��ȡ����Ӧ��EΪ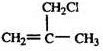 ��E����ˮ��õ���F��GΪȩ�Һ���˫��������д����ṹΪCH2=C��CH3��-CHO��������Ӧ�ߵõ�����HΪCH2=C��CH3��-COOH��H��CH3OH�õ���IΪCH2=C��CH3��-COOCH3����JΪ�Ӿ۷�Ӧ�IJ��Ϊ
��E����ˮ��õ���F��GΪȩ�Һ���˫��������д����ṹΪCH2=C��CH3��-CHO��������Ӧ�ߵõ�����HΪCH2=C��CH3��-COOH��H��CH3OH�õ���IΪCH2=C��CH3��-COOCH3����JΪ�Ӿ۷�Ӧ�IJ��Ϊ ��
��
��1�������Ϸ�����֪BΪ��CH3��2CHCOOH��D�Ľṹ��ʽΪ����CH3��2C�TCH2��
�ʴ�Ϊ����CH3��2CHCOOH����CH3��2C�TCH2��
��2�������Ϸ�����֪G�Ľṹ��ʽΪ��CH2=C��CH3��-CHO��
�ʴ�Ϊ��CH2=C��CH3��-CHO��
��3����Ӧ��Ϊ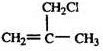 ��ˮ�ⷴӦ����Ӧ�ķ���ʽΪ
��ˮ�ⷴӦ����Ӧ�ķ���ʽΪ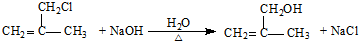 ��
��
��Ӧ��ΪCH2=C��CH3��-COOCH3�ļӾ۷�Ӧ����Ӧ�ķ���ʽΪ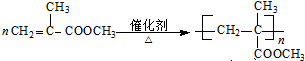 ��
��
�ʴ�Ϊ��ˮ�ⷴӦ���Ӿ۷�Ӧ��
��4���ɷ�Ӧ���������ŵı仯��֪����Ӧ��Ϊˮ�ⷴӦ����Ӧ��Ϊ�ӳɷ�Ӧ����Ϊ������Ӧ��
�ʴ�Ϊ��ˮ�ⷴӦ���ӳɷ�Ӧ��������Ӧ��
��5��HΪCH2=C��CH3��-COOH����H������ͬ�����ŵ�H��ͬ���칹����CH2=CHCH2COOH ��CH3CH=CHCOOH��CH3CH=CHCOOH��˳���칹��������ͬ���칹��Ľṹ��ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
���� ���⿼���л�����ƶϣ���Ŀ�Ѷ��еȣ����ؼ����ҽ����ͻ�ƿڣ������ۣ�������A��B+C��ˮ�ⷴӦ�������ж�A��B��C�ֱ�����������ʹ������ɣ�1������ȷ��B��C�Ľṹ���Դ˿��ƶ��������ʣ�ע���л�������ŵĽṹ�����ʣ�Ϊ��ȷ��������Ŀ�Ĺؼ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | �����������Ҵ���������Ӧ����H��0������������Ũ���Ტ���ȣ��÷�Ӧ�ķ�Ӧ���ʺ�ƽ�ⳣ�������� | |
| B�� | ���ᱡ�ɴ�������ͼ ���ܷ���ˮ�⡢��������ȥ��Ӧ���Լ��������ļӳɷ�Ӧ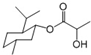 | |
| C�� | 0.1mol��ϩ���к���˫������ĿΪ0.1NA | |
| D�� | ��ȩ�ͱ�ϩȩ��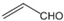 ������ͬϵ�������������ַ�Ӧ��IJ�����ͬϵ�� ������ͬϵ�������������ַ�Ӧ��IJ�����ͬϵ�� |
| A�� | ������ҩ����ϩ�ƷӵĽṹ��ʽΪ��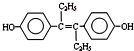 �����ķ���ʽ�ǣ�C18H20O2 �����ķ���ʽ�ǣ�C18H20O2 | |
| B�� | �������ļ��顢��ϩ���Ҵ��ֱ���ȼ�գ������������������μ��� | |
| C�� | �����ᣨ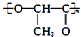 �������ɵ���֮��ͨ���Ӿ۷�Ӧ�ϳɵ� �������ɵ���֮��ͨ���Ӿ۷�Ӧ�ϳɵ� | |
| D�� | ʵ��֤ʵ ��ʹ������Ȼ�̼��Һ��ɫ��˵���÷����д���̼̼˫���� ��ʹ������Ȼ�̼��Һ��ɫ��˵���÷����д���̼̼˫���� |
| A�� | ��ϩ�ƾ���ϩ | B�� | �������Ҵ����������� | ||
| C�� | �Ҵ�����ȩ | D�� | �������ȩ�Ʒ�ȩ��֬ |
| A�� | �õ����þΪ������������ԭ��Ӧ | |
| B�� | ��ع���ʱ��OH-�������ƶ� | |
| C�� | �õ�ص��ܷ�ӦΪ��Mg+ClO-+H2O=Mg��OH��2��+Cl- | |
| D�� | ��ع���ʱ��������Χ��Һ��pH�����ϱ�С |
| A�� | ��Ϊs�������״�����εģ�����s����������Բ���˶� | |
| B�� | 3px��3py��3pz�IJ���֮�����������е��ӣ���̬����������ͬ | |
| C�� | ԭ�ӹ���͵����ƶ���������������������˶�״̬�� | |
| D�� | ������ͼ�ϵ�ÿһ���㶼����һ������ |