��Ŀ����
�����Ȼ�ѧ����ʽ�У���H�ľ���ֵ�ܱ�ʾ��ȼ���ȼ���ȵ���(����)
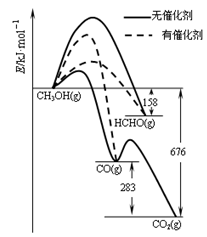
A��C(s)��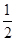 O2(g)===CO(g)����H����110.5 kJ��mol��1 O2(g)===CO(g)����H����110.5 kJ��mol��1 |
| B��CH4(g)��2O2(g)===CO2(g)��2H2O(g)��H����802.3 kJ��mol��1 |
| C��2H2(g)��O2(g)===2H2O(l)����H����571.6 kJ��mol��1 |
D��CO(g)��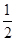 O2(g)===CO2(g)����H����283 kJ��mol��1 O2(g)===CO2(g)����H����283 kJ��mol��1 |
D
�������������ȼ��������һ�������£�1mol��ȼ����ȫȼ�������ȶ���������ʱ���ų������������Ը����Ȼ�ѧ����ʽ��֪ѡ��D���Ա�ʾCO��ȼ���ȡ�A��̼���ȶ�������CO2��B��ˮ���ȶ�״̬��Һ̬��C�����������ʵ���Ӧ����1mol����ѡD��
���㣺����ȼ���ȵ��ж�
����������Ĺؼ�����ȷȼ�յĺ��壬�ر����ж����ݣ�Ȼ��ͽ��������Ȼ�ѧ����ʽ������á��жϼ��ɣ��ѶȲ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д����и����е������������ã���Ӧ����(�¶Ȼ��߷�Ӧ������)�ı䣬���������������ı����(����)
| A��Na��O2 | B��NaOH��CO2 |
| C��Na2O2��CO2 | D��AlCl3��NaOH |
���з�Ӧ����������ԭ��Ӧ���������ȷ�Ӧ���ǣ�����
| A����Ƭ��ϡH2SO4�ķ�Ӧ | B��Ba(OH)2��8H2O��NH4Cl�ķ�Ӧ |
| C�����ȵ�̿��CO2�ķ�Ӧ | D��������O2�е�ȼ�շ�Ӧ |
�Ҵ����ӽṹ�и��ֻ�ѧ��������ʾ�������Ҵ��ڸ��ַ�Ӧ�жϼ���˵������ȷ��Ϊ
| A�������Ṳ��ʱ�����Ѣټ� |
| B���ͽ����Ʒ�Ӧʱ�����ٶ��� |
| C����P2O5����ʱ�����ڢݶ��� |
| D����ͭ���º�������Ӧʱ�����٢ݶ��� |
��֪��ӦA2(g)��B2(g)=2AB(g)���Ͽ�1molA2�еĻ�ѧ�����ĵ�����ΪQ1 kJ���Ͽ�1molB2�еĻ�ѧ�����ĵ�����ΪQ2 kJ������1molAB�еĻ�ѧ���ͷŵ�����ΪQ3kJ��Q1��Q2��Q3�������㣩��������˵����ȷ���� �� ��
| A����A2��B2��������֮�ʹ������ɵ�2AB������������Ӧ���� |
| B����A2��B2��������֮��С�����ɵ�2AB������������Ӧ���� |
| C�����÷�ӦΪ���ȷ�Ӧ����Q1��Q2 < Q3 |
| D�����÷�ӦΪ���ȷ�Ӧ����Q1��Q2 < Q3 |
(16��) ̼���仯�����й㷺����;��
��1����ӦC(s)�� H2O(g)  CO(g) ��H2(g) ��H=" +131.3" kJ?mol-1���ﵽƽ����������ʱ���������������H2���ʵĴ�ʩ�� ��
CO(g) ��H2(g) ��H=" +131.3" kJ?mol-1���ﵽƽ����������ʱ���������������H2���ʵĴ�ʩ�� ��
| A������̼�������� | B�������¶� | C����CO���ռ���ȥCO | D��������� |
 2CO��g�� ��H=+172.5kJ?mol-1
2CO��g�� ��H=+172.5kJ?mol-1��Ӧ CO��g��+H2O��g��
 CO2��g��+H2��g�� �ġ�H= kJ?mol-1��
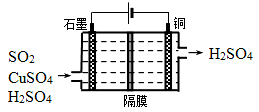
CO2��g��+H2��g�� �ġ�H= kJ?mol-1����3��CO��H2һ�������·�Ӧ���ɼ״���CH3OH�����״���һ��ȼ�ϣ������ü״����һ��ȼ�ϵ�أ���ϡ�������������Һ�����ʯī���缫���õ�ظ�����ӦʽΪ ��
��4����һ���¶��£���CO(g)��H2O(g)��0.16 mol�ֱ�ͨ�뵽���Ϊ2.0L�ĺ����ܱ������У��������·�Ӧ��CO(g)��H2O(g)
 CO2(g)��H2(g)���õ��������ݣ�
CO2(g)��H2(g)���õ��������ݣ�| t / min | 2 | 4 | 7 | 9 |
| n(H2O)/mol | 0.12 | 0.11 | 0.10 | 0.10 |
�ڸ��¶��£��˷�Ӧ��ƽ�ⳣ��K=______ _____��
�������������䣬�ٳ���0.1mol CO��0.1mol H2O(g)��ƽ��ʱCO���������______(���������С�����䡱)��
��16�֣�����β���е�CO��NOX�Ѿ���Ϊ��������Ҫ��Ⱦ�ʹ��ϡ���ȴ����ܽ�CO��NOX��̼�⻯����ת���������ʣ��Ӷ���������β����Ⱦ��
��1����֪��N2(g)��O2(g)��2NO(g) ��H1 ��
2C(s)��O2(g)��2CO(g) ��H2 ��
C(s)��O2(g)��CO2(g) ��H3 ��
д��NO��CO��ת����N2��CO2���Ȼ�ѧ����ʽ����Ӧ���á�H1����H2����H3��ʾ���� ��
��2�����ݻ���ͬ�������ܱ������ڣ�װ�е�����ij�ִ��������ֱ����ͬ����NOx��C3H6���ڲ�ͬ�¶��£�ͬʱ�������·�Ӧ��
18NO(g)��2C3H6(g) 9N2(g)��6CO2(g)��6H2O(g)��
9N2(g)��6CO2(g)��6H2O(g)��
18NO2(g)��4C3H6(g) 9N2(g)��12CO2(g)��12H2O(g)��
9N2(g)��12CO2(g)��12H2O(g)��
���ֱ���t��ʱ�ⶨ����NOX��ת���ʣ����ͼ������ͼ��ʾ��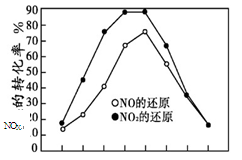
�� ��ͼ�п��Եó��Ľ�����
����һ����ͬ�¶��£� ��
���۶��� ��
�� ����NO2��C3H6��Ӧ��ƽ������NO2
ת���ʵĴ�ʩ�� �������ţ�
| A��������� | B�������¶� |
| C�������H2O(g) | D������ѹǿ |
���ַ�Ӧ����̼��Ѫ�쵰�ף�Hb��CO������37��ʱ��CO��Hb��O2
 O2��Hb��CO K��220
O2��Hb��CO K��220ʵ�������Hb��CO��Ũ�ȼ�ʹֻ��Hb��O2Ũ�ȵ�2%��Ҳ������˵��������ˡ����̺��ƽ��ʱ����β��Ŀ�����CO��O2��Ũ�ȷֱ�Ϊ10��6 mol��L��1��10��2 mol��L��1�����ʣ����̻�����˵����������𣿣�д��������̣�