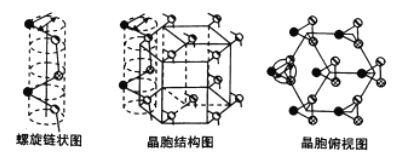
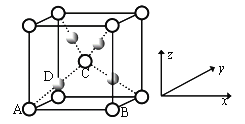
��Ŀ����
����Ŀ��A��G����ѧ��ѧ�������ʣ�A��DΪ���ʣ�G�Ǻ�AԪ�ص��������塣��֪��A(s)+B(g)=C(g)+D(g) ��H= +131.4 kJmol-1��ijͬѧʵ���֪��4 g A����������Ӧ����43.8 kJ��������
��1��д��AԪ�ص�����________��
��2������֪��
A(s)+O2(g)=G(g)��H= ��393.6 kJmol-1
C(g)+ O2(g)=G(g)��H=��283 kJmol-1
D(g)+ O2(g)=B(g)��H=��242 kJmol-1�ɴ��жϡ���Ϊ283 kJmol-1+242 kJmol-1>393.6 kJmol-1������Aȼ��ʱ������B���Էų����������������˵���Ƿ���ȷ��___________��������_____________________��
��3��д��A+O2��C���Ȼ�ѧ����ʽ��__________________________��
���𰸡� ̼ ����ȷ 1 mol A��O2ֱ��ȼ�շų�������Ϊ393.6 kJ����1 mol A����B��Ӧ����C ��D��C��D����O2��Ӧ����������-131.4 kJ+283 kJ+242 kJ=393.6 kJ��������ͬ C(s)+1/2O2(g)====CO(g) ��H=��110.6 kJmol-1
��������A��DΪ���ʣ�G�Ǻ�AԪ�ص��������壬��Ҫ��֪AΪ�ǽ���Ԫ�أ�A��DΪ���ʣ���֪A��B�������û���Ӧ����A��C������������Ӧ���ɺ�A���������壬���ϴ���������ѧ������Ԫ����̼��������A��B�û��IJ���DҲ���Ժ�������Ӧ������Aֻ��Ϊ̼Ԫ�أ�BΪˮ��CΪһ����̼��DΪ������GΪ������̼��
��1��AΪ̼Ԫ�أ��ʴ�Ϊ��̼��
��2����Ϊ1molA��O2ֱ��ȼ�շų�������Ϊ393.6kJ����1molA����B��Ӧ����C��D��C��D����O2��Ӧ�����ų�������-131.4kJ+283kJ+242kJ=393.6kJ��������ͬ��������Ŀ�е�˵������ȷ���ʴ�Ϊ������ȷ����Ϊ1molA��O2ֱ��ȼ�շų�������Ϊ393.6kJ����1molA����B��Ӧ����C��D��C��D����O2��Ӧ�����ų�������-131.4kJ+283kJ+242kJ=393.6kJ��������ͬ��
��3��C��s��+![]() O2��g���TCO��g�����Կ����Ƿ�Ӧ��A��s��+O2��g���TG��g����H=-393.6kJmol-1��ȥ��Ӧ��C��g��+
O2��g���TCO��g�����Կ����Ƿ�Ӧ��A��s��+O2��g���TG��g����H=-393.6kJmol-1��ȥ��Ӧ��C��g��+![]() O2��g���TG��g����H=-283kJmol-1�õ������ݸ�˹���ɿ�֪����H�T-393.6kJmol-1-��-283kJmol-1��=-110.6kJmol-1�����Ȼ�ѧ����ʽΪ��C��s��+
O2��g���TG��g����H=-283kJmol-1�õ������ݸ�˹���ɿ�֪����H�T-393.6kJmol-1-��-283kJmol-1��=-110.6kJmol-1�����Ȼ�ѧ����ʽΪ��C��s��+![]() O2��g���TCO��g����H=-110.6kJmol-1���ʴ�Ϊ��C��s��+
O2��g���TCO��g����H=-110.6kJmol-1���ʴ�Ϊ��C��s��+![]() O2��g���TCO��g����H=-110.6kJmol-1��
O2��g���TCO��g����H=-110.6kJmol-1��

 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д�