��Ŀ����
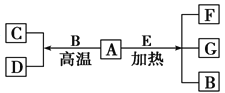
����Ŀ����1����������������뵪ѭ�����ϳɰ���ҵ���Dz�����ֶ�֮һ������Ȼ��Ϊԭ�Ϻϳɰ����µ��������ʵķ�������������ȾС���ɱ��͵�����ŵ�������̴�����ͼ1��ʾ��

����д������Ȼ���Ʊ������Ļ�ѧ����ʽ��_________________________________��
��д���ϳ�����[CO (NH2) 2]��Ӧ�Ļ�ѧ����ʽ��__________________________��
��д��O2��NH3���д�������������Ӧ����NH4NO3��H2O�Ļ�ѧ����ʽ��______��
��ÿ����1molNH4NO3������ҪNH3______mol����Ҫ������ЩNH3��������ҪCH4____mol��
��2����ѧ���ѻ�ü��������о������N4���ӣ���ṹΪ�������壨��ͼ2������֪����1molN-N������193kJ����������1molN��N������941kJ��������1molN4����ת��ΪN2ʱҪ�ͷ�______kJ������
���𰸡� CH4+2H2O![]() CO2+4H2 CO2+2NH3
CO2+4H2 CO2+2NH3![]() CO��NH2��2+H2O 2NH3+2O2
CO��NH2��2+H2O 2NH3+2O2![]() NH4NO3+H2O 2 0.75 724
NH4NO3+H2O 2 0.75 724
����������1����CH4ת��������������CO2��H2������ʽΪ��CH4+2H2O![]() CO2+4H2���ʴ�Ϊ��CH4+2H2O
CO2+4H2���ʴ�Ϊ��CH4+2H2O![]() CO2+4H2��
CO2+4H2��
��������̼�Ͱ�����Ӧ�������أ�����ԭ���غ㣺CO2+2NH3![]() CO��NH2��2+H2O���ʴ�Ϊ��CO2+2NH3
CO��NH2��2+H2O���ʴ�Ϊ��CO2+2NH3![]() CO��NH2��2+H2O��
CO��NH2��2+H2O��
��O2��NH3��Ӧ����NH4NO3������ԭ���غ㷴Ӧ�Ļ�ѧ����ʽΪ2NH3+2O2![]() NH4NO3+H2O���ʴ�Ϊ��2NH3+2O2
NH4NO3+H2O���ʴ�Ϊ��2NH3+2O2![]() NH4NO3+H2O��
NH4NO3+H2O��
��NH4NO3��2NH3��ÿ����1mol NH4NO3������Ҫ2molNH3�����ݷ�ӦCH4+2H2O![]() CO2+4H2��N2+3H2
CO2+4H2��N2+3H2![]() 2NH3����֪����2molNH3��Ҫ����0.75mol���ʴ�Ϊ��2��0.75��
2NH3����֪����2molNH3��Ҫ����0.75mol���ʴ�Ϊ��2��0.75��
��2���ӽṹͼ�пɿ�����һ��N4�����к���6��N-N�������ݷ�Ӧ�ȵ��ڷ�Ӧ����ܼ��ܼ���������ܼ��ܣ���N4��g��=2N2��g����H������H=6��193 kJmol-1-2��941 kJmol-1=-724 kJmol-1���ʴ�Ϊ��724��