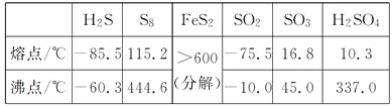
��Ŀ����
����Ŀ����ͼװ�ã�����һ�ֿɳ���أ�װ�ã���Ϊ���ء�
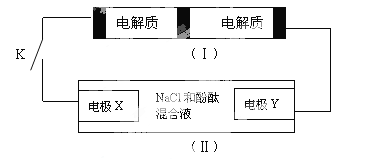
װ�ã������ӽ���Ĥֻ����Na+ͨ������֪��س�ŵ�Ļ�ѧ����ʽΪ2Na2S2+NaBr3����![]() Na2S4+3NaBr�����պϿ���Kʱ��X�缫������Һ��졣����˵����ȷ����
Na2S4+3NaBr�����պϿ���Kʱ��X�缫������Һ��졣����˵����ȷ����
A. �պϿ���Kʱ�������Ӵ��ҵ���ͨ�����ӽ���Ĥ
B. �պϿ���Kʱ��������ӦʽΪ��3NaBr-2e-=NaBr3+2Na+
C. �պϿ���Kʱ��X�缫��ӦʽΪ��2Cl--2e-=Cl2��
D. �պϿ���Kʱ������0.1molNa+ͨ�����ӽ���Ĥ��X�缫��������״��������1.12L
���𰸡�D
��������
���պϿ���Kʱ��X��������Һ���ɫ��˵��X������OH�����ӣ�Ϊ���ص�������������ӦΪ2H2O+2e����H2��+2OH�������ݵ�س䡢�ŵ�Ļ�ѧ��Ӧ����ʽΪ2Na2S2+NaBr3![]() Na2S4+3NaBr��֪��������ӦΪ2Na2S2��2e����2Na++Na2S4��������ӦΪNaBr3+2Na++2e����3NaBr��Y��Ϊ���ص��������缫��ӦΪ2Cl�D�D2e����Cl2�����Դ˽����⡣
Na2S4+3NaBr��֪��������ӦΪ2Na2S2��2e����2Na++Na2S4��������ӦΪNaBr3+2Na++2e����3NaBr��Y��Ϊ���ص��������缫��ӦΪ2Cl�D�D2e����Cl2�����Դ˽����⡣
A��ԭ������������������ƶ�����պ�Kʱ�������Ӵ�����ͨ�����ӽ���Ĥ����A����
B���պ�Kʱ����������������Ӧ���缫��ӦΪ2Na2S2��2e����2Na++Na2S4����B����
C���պ�Kʱ��X��������Һ���ɫ��˵��X������OH�����ӣ�Ϊ���ص�������������ӦΪ2H2O+2e����H2��+2OH������C����
D���պ�Kʱ����0.1molNa+ͨ�����ӽ���Ĥ��˵����0.1mol����ת�ƣ�����������0.05molH2����״�������Ϊ0.05mol��22.4L/mol��1.12L����D��ȷ��
��ѡD��

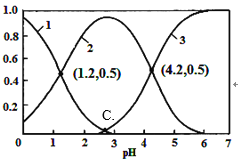
����Ŀ��������25 ��ʱijЩ�ε��ܶȻ�����������ĵ���ƽ�ⳣ��������˵����ȷ����
��ѧʽ | AgCl | Ag2CrO4 | CH3COOH | HClO | H2CO3 |
Ksp��Ka | Ksp=1.8��10��10 | Ksp=9.0��10��12 | Ka=1.8��10��5 | Ka=3.0��10��8 | Ka1=4.1��10��7 Ka2=5.6��10��11 |
A. H2CO3��HCO3����CH3COO����ClO�� ����Һ�п��Դ�������
B. �������Ũ�ȵ�CH3COONa��NaClO������������CH3COONa ��NaClO
C. ��Ũ�Ⱦ�Ϊ1.0��10��3 mol��L��1��KCl��K2CrO4�����Һ�еμ�1.0��10��3 mol��L��1��AgNO3��Һ��CrO42�D���γɳ���
D. ��0.1 mol��L��1 CH3COOH��Һ�еμ�NaOH��Һ����c(CH3COOH):c(CH3COO��)��5��9����ʱ��Һ��pH=5
����Ŀ��POC13�������뵼����Ӽ������άԭ�ϣ�ʵ�����Ʊ�POC13���ⶨ��Ʒ������ʵ��������£�
I.ʵ�����Ʊ�POC13��������������Һ̬PCl3����ȡPOC13��ʵ��װ��(���ȼ��г�������)����ͼ:
���ϣ���Ag��+SCN��=AgSCN�� Ksp(AgCl)>Ksp(AgSCN)��
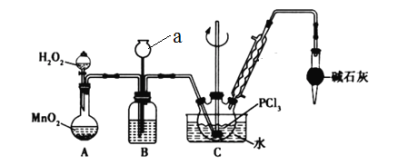
��PCl3��POC13�������Ϣ���±���
���� | �۵�/�� | �е�/�� | ��Է������� | ���� |
PCl3 | -112.0 | 76.0 | 137.5 | �����ܣ���Ϊ��ɫҺ�壬��ˮ�����ҷ�Ӧ���ɺ�������Ȼ��� |
POC13 | 2.0 | 106.0 | 153.5 |
(1)����a������____________________��
(2)B����ʢ���Լ���________������ܵ�������_____________________��
(3)POC13��ˮ��Ӧ�Ļ�ѧ����ʽΪ____________________________��
(4)��Ӧ�¶�Ҫ������60~65�棬ԭ���ǣ�____________________________��
II.�ⶨPOC13��Ʒ�ĺ�����ʵ�鲽�裺
���Ʊ�POC13ʵ�����������ƿ�е�Һ����ȴ�����£�ȷ��ȡ29.1g��Ʒ������ʢ��60.00 mL����ˮ��ˮ��ƿ��ҡ������ȫˮ�⣬��ˮ��Һ���100.00 mL��Һ��
��ȡ10.00 mL��Һ����ƿ�У�����20.00 mL 3.5mol/L AgNO3����Һ��
�ۼ�����������������ҡ����ʹ�������汻�л��︲�ǡ�
����XΪָʾ������1.00mol/LKSCN��Һ�ζ�����AgNO3��Һ���ﵽ�ζ��յ�ʱ����ȥ10.00mLKSCN��Һ��
(5)�������X����ѡ��___________________ ��
(6)����������������������ᵼ�²������______(��ƫ�ߣ�ƫ�ͣ�����Ӱ��)
(7)��Ӧ������POC13�������ٷֺ���Ϊ___________________�� ���ζ��յ㣬��ȡKSCN��Һ���ӿ̶��ߣ����������____________(��ƫ�ߣ�ƫ�ͣ�����Ӱ��)