题目内容
【题目】用化学知识填空:
(1)丙烷通过脱氢反应可得丙烯。
已知:①C3H8(g)= CH4(g)+C2H2(g)+H2(g) ΔH1=+156.6 kJ·mol![]()
②C3H6(g)= CH4(g)+C2H2(g) ΔH2=+32.4 kJ·mol![]()
则相同条件下,反应C3H8(g)=== C3H6(g)+H2(g)的ΔH=__________kJ·mol![]() 。
。
(2)将煤转化为水煤气是通过化学方法将煤转化为洁净燃料的方法之一。煤转化为水煤气的主要化学反应为C(s)+H2O(g) ![]() CO(g)+H2(g)。C(s)、CO(g)和H2(g)完全燃烧的热化学方程式分别为
CO(g)+H2(g)。C(s)、CO(g)和H2(g)完全燃烧的热化学方程式分别为
C(s)+O2(g)===CO2(g) ΔH1=-393.5 kJ·mol![]()
H2(g)+![]() O2(g)===H2O(g) ΔH2=-242.0 kJ·mol
O2(g)===H2O(g) ΔH2=-242.0 kJ·mol![]()
CO(g)+![]() O2(g)===CO2(g) ΔH3=-283.0 kJ·mol
O2(g)===CO2(g) ΔH3=-283.0 kJ·mol![]()
写出C(s)与水蒸气反应生成CO和H2的热化学方程式: ______________________。
(3)3mol甲烷燃烧时,生成液态水和二氧化碳,同时放出2 670.9kJ的热量,写出甲烷燃烧热的热化学方程式_________________________________________________
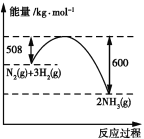
(4)如图是298 K、101 kPa时,N2与H2反应过程中能量变化的曲线图。该反应的热化学方程式为____________。
(5)已知:H2(g)+Cl2(g)===2HCl(g) ΔH=-185 kJ·mol![]() Cl—Cl 的键能为247 kJ·mol
Cl—Cl 的键能为247 kJ·mol![]() ,H—H的键能为436kJ·mol
,H—H的键能为436kJ·mol![]() 则H—Cl的键能为______________kJ·mol
则H—Cl的键能为______________kJ·mol![]()
【答案】+124.2 C(s)+H2O(g) === CO(g) +H2(g) ΔH=+131.5 kJ·mol-1 CH4(g)+2O2(g) === CO2(g) +2 H2O (l) ΔH=-890.3 kJ·mol-1 N2(g)+3H2(g) 2NH3(g) △H=-92 kJ·mol-1 434
【解析】
从盖斯定律、燃烧热的定义、反应热与焓变的关系角度进行分析;
(1)利用盖斯定律,根据目标反应方程式,因此有①-②得出△H=△H1-△H2=+156.6kJ·mol-1-(+32. 4kJ·mol-1)=+124.2kJ·mol-1,
故答案为+124.2kJ·mol-1;
(2)C(s)+H2O(g) ![]() CO(g)+H2(g) ①,H2(g)+
CO(g)+H2(g) ①,H2(g)+![]() O2(g)===H2O(g) ②,CO(g)+
O2(g)===H2O(g) ②,CO(g)+![]() O2(g)===CO2(g) ③,利用盖斯定律,得出①-②-③得出:△H=△H1-△H2-△H3=(-393.5kJ·mol-1)-(-242.0kJ·mol-1-283.0kJ·mol-1)=+131.5kJ·mol-1,则热化学反应方程式为C(s)+H2O(g) = CO(g) +H2(g) ΔH=+131.5 kJ·mol-1,
O2(g)===CO2(g) ③,利用盖斯定律,得出①-②-③得出:△H=△H1-△H2-△H3=(-393.5kJ·mol-1)-(-242.0kJ·mol-1-283.0kJ·mol-1)=+131.5kJ·mol-1,则热化学反应方程式为C(s)+H2O(g) = CO(g) +H2(g) ΔH=+131.5 kJ·mol-1,
故答案为C(s)+H2O(g) = CO(g) +H2(g) ΔH=+131.5 kJ·mol-1;
(3)根据燃烧热的定义,得出1mol甲烷完全燃烧放出的热量为![]() ==890.3kJ·mol-1,因此甲烷燃烧热的热化学方程式为CH4(g)+2O2(g) = CO2(g) +2 H2O (l) ΔH=-890.3 kJ·mol-1,
==890.3kJ·mol-1,因此甲烷燃烧热的热化学方程式为CH4(g)+2O2(g) = CO2(g) +2 H2O (l) ΔH=-890.3 kJ·mol-1,
故答案为CH4(g)+2O2(g) = CO2(g) +2 H2O (l) ΔH=-890.3 kJ·mol-1;
(4)根据图像,反应物总能量大于生成物的总能量,即该反应为放热反应,因此△H=(508kJ·mol-1-600kJ·mol-1)=92kJ·mol-1,热化学反应方程式为N2(g)+3H2(g) ![]() 2NH3(g) △H=-92 kJ·mol-1,
2NH3(g) △H=-92 kJ·mol-1,
故答案为N2(g)+3H2(g) ![]() 2NH3(g) △H=-92 kJ·mol-1;
2NH3(g) △H=-92 kJ·mol-1;
(5)利用△H=反应物键能总和-生成物键能总和,令H-Cl的键能为akJ·mol-1,则有:436kJ·mol-1+247kJ·mol-1-2akJ·mol-1=-185kJ·mol-1,解得a=434kJ·mol-1,
故答案为434kJ·mol-1。