��Ŀ����
����Ŀ��CO2����Դ����������Ч����CO2�ŷţ��������̼��Դ��CaO���ڽϸ��¶��²���CO2���ڸ����¶��½�������CO2�ͷ����á�CaC2O4��H2O�ȷֽ���Ʊ�CaO��CaC2O4��H2O�������¹����й���������仯��ͼ��

��д��400~600 �淶Χ�ڷֽⷴӦ�Ļ�ѧ����ʽ��________��
����CaCO3�ȷֽ��Ʊ���CaO��ȣ�CaC2O4��H2O�ȷֽ��Ʊ���CaO���и��õ�CO2�������ܣ���ԭ����________��
���𰸡�CaC2O4![]() CaCO3+CO�� CaC2O4��H2O�ȷֽ�ų���������壬�Ƶõ�CaO�������ɶ��
CaCO3+CO�� CaC2O4��H2O�ȷֽ�ų���������壬�Ƶõ�CaO�������ɶ��
��������
CaC2O4��H2O�ȷֽ��ȷֽ����ɲ���ƺ�ˮ��0200�������������仯146128��18����Ӧ�Ļ�ѧ����ʽ��0200�������ķ�Ӧ��CaC2O4��H2O![]() CaC2O4��H2O��400600����������128100��28������Ʒֽ�������һ����̼��̼��ƣ��Ƿ�����ӦCaC2O4
CaC2O4��H2O��400600����������128100��28������Ʒֽ�������һ����̼��̼��ƣ��Ƿ�����ӦCaC2O4![]() CaCO3��CO����8001000���������仯��10056��44����̼��Ʒֽ����������ƺͶ�����̼����Ӧ�Ļ�ѧ����ʽ��CaCO3
CaCO3��CO����8001000���������仯��10056��44����̼��Ʒֽ����������ƺͶ�����̼����Ӧ�Ļ�ѧ����ʽ��CaCO3![]() CaO��CO2�����ݴ˷����жϣ�
CaO��CO2�����ݴ˷����жϣ�
������������֪400��600����Χ�ڷֽⷴӦΪ����Ʒֽ�����̼��ƺ�һ����̼��
��CaC2O4H2O�ȷֽ�ų���������塣
CaC2O4��H2O�ȷֽ��ȷֽ����ɲ���ƺ�ˮ��0200�������������仯146128��18����Ӧ�Ļ�ѧ����ʽ��0200�������ķ�Ӧ��CaC2O4��H2O![]() CaC2O4��H2O��400600����������128100��28������Ʒֽ�������һ����̼��̼��ƣ��Ƿ�����ӦCaC2O4
CaC2O4��H2O��400600����������128100��28������Ʒֽ�������һ����̼��̼��ƣ��Ƿ�����ӦCaC2O4![]() CaCO3��CO����8001000���������仯��10056��44����̼��Ʒֽ����������ƺͶ�����̼����Ӧ�Ļ�ѧ����ʽ��CaCO3
CaCO3��CO����8001000���������仯��10056��44����̼��Ʒֽ����������ƺͶ�����̼����Ӧ�Ļ�ѧ����ʽ��CaCO3![]() CaO��CO2�����ݴ˷����жϣ�
CaO��CO2�����ݴ˷����жϣ�
��400600����������128100��28������Ʒֽ�������һ����̼��̼��ƣ��Ƿ�����ӦCaC2O4![]() CaCO3��CO�����ʴ�Ϊ��CaC2O4
CaCO3��CO�����ʴ�Ϊ��CaC2O4![]() CaCO3+CO����
CaCO3+CO����
��CaC2O4H2O�ȷֽ��Ʊ���CaO���и��õ�CO2�������ܣ���ԭ���ǣ�CaC2O4H2O�ȷֽ�ų���������壬�Ƶõ������Ƹ������ɶ�ף��ʴ�Ϊ��CaC2O4��H2O�ȷֽ�ų���������壬�Ƶõ�CaO�������ɶ�ס�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ����![]() �ǻ���������
�ǻ���������![]() �������ķ�Ӧ���£�HOCH2CH2CH2COOH
�������ķ�Ӧ���£�HOCH2CH2CH2COOH![]() +H2O
+H2O
��298K�£�![]() �ǻ�����ˮ��Һ�ij�ʼŨ��Ϊ
�ǻ�����ˮ��Һ�ij�ʼŨ��Ϊ![]() �����
�����![]() ��������Ũ����ʱ��仯�����������ʾ���ش��������⣺
��������Ũ����ʱ��仯�����������ʾ���ش��������⣺
| 21 | 50 | 80 | 100 | 120 | 160 | 220 |
|
| 0.024 | 0.050 | 0.071 | 0.081 | 0.090 | 0.104 | 0.116 | 0.132 |
(1)�÷�Ӧ��50��80min�ڵ�ƽ����Ӧ����Ϊ_____![]() ��
��
(2)120minʱ![]() �ǻ������ת����Ϊ______��
�ǻ������ת����Ϊ______��
(3)298Kʱ�÷�Ӧ��ƽ�ⳣ��![]() _____��
_____��
(4)Ϊ���![]() �ǻ������ƽ��ת���ʣ����ʵ����Ʒ�Ӧ�¶��⣬���ɲ�ȡ�Ĵ�ʩ��______��
�ǻ������ƽ��ת���ʣ����ʵ����Ʒ�Ӧ�¶��⣬���ɲ�ȡ�Ĵ�ʩ��______��
����Ŀ��ijͬѧ��������ʵ�飺
���� | ���� |
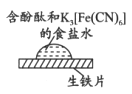
ȡһ���ĥ��������Ƭ����������1�κ���̪��K3[Fe��CN��6]��ʳ��ˮ
| ����һ��ʱ�������Ƭ�ϳ�����ͼ��ʾ���ߺۡ�����Ե��Ϊ��ɫ����������Ϊ��ɫ������ɫ�����紦��������
|
���ж�ʵ�������������ȷ����
A. ������Ϊ��ɫ��ԭ������ʧ���ӱ�������Fe2����K3[Fe��CN��6]��Ӧ������ɫ����
B. ��Ե��Ϊ��ɫ��ԭ���Ƿ���������ʴ��������OH����ʹ��Һ��c��OH����>c��H����
C. ��Ե��Ϊ��ɫ��ԭ���Ƿ������ⸯʴ��������H����������ˮ�ĵ���ƽ�⣬ʹc��OH����>c��H����
D. ���紦���������ԭ����4Fe2����8OH����O2��2H2O��4Fe��OH��3��Fe��OH��3�ֽ�õ�����