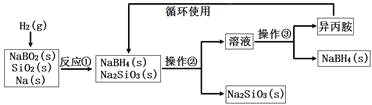
��Ŀ����
����Ŀ���о�SO2��CO�ȴ�����Ⱦ����Ĵ���������Ҫ���塣
��1�������Ƽ�ѭ�������ѳ������е�SO2�����Ƽ�ѭ�����У���Na2SO3��Һ��Ϊ����Һ����SO2�Ĺ����У�pH��n��SO![]() ����n��HSO
����n��HSO![]() ���仯��ϵ���±���
���仯��ϵ���±���
n��SO | 91��9 | 1��1 | 9��91 |
pH | 8��2 | 7��2 | 6��2 |
�����ϱ��жϣ�NaHSO3��Һ��____________�ԣ���ƽ��ԭ�����ͣ�_____________________��
�ڵ�����Һ������ʱ����Һ������Ũ�ȹ�ϵ��ȷ������ѡ����ĸ��___________________��
a��c��Na������2c��SO![]() ����c��HSO
����c��HSO![]() ��
��
b��c��Na����>c��HSO![]() ��>c��SO
��>c��SO![]() ��>c��H������c��OH����
��>c��H������c��OH����
c��c��Na������c��H������c��SO![]() ����c��HSO
����c��HSO![]() ����c��OH����
����c��OH����
��2����ij��Һ�к�3mol Na2SO3������һ������ϡ���ᣬǡ��ʹ��Һ��Cl����HSO![]() �����ʵ���֮��Ϊ2��1��������������HCl�����ʵ�������_____________mol��
�����ʵ���֮��Ϊ2��1��������������HCl�����ʵ�������_____________mol��
��3��CO�����ںϳ�CH3OH����Ӧ����ʽΪ��CO��g��+2H2��g��![]() CH3OH��g�� ,��һ���¶�ѹǿ�£����ݻ�Ϊ2L���ܱ�������ͨ��0��2molCO��0��4molH2����ƽ��ʱCO��ת����50%������¶��µ�ƽ�ⳣ��Ϊ ���ټ���1��0molCO�����´ﵽƽ�⣬��CO��ת���� ������������䡱��С����CH3OH��������� ������������䡱��С������
CH3OH��g�� ,��һ���¶�ѹǿ�£����ݻ�Ϊ2L���ܱ�������ͨ��0��2molCO��0��4molH2����ƽ��ʱCO��ת����50%������¶��µ�ƽ�ⳣ��Ϊ ���ټ���1��0molCO�����´ﵽƽ�⣬��CO��ת���� ������������䡱��С����CH3OH��������� ������������䡱��С������
��4����0��02mol/LNa2SO4��Һ��ijŨ��BaCl2��Һ�������ϣ�������BaSO4��������ԭBaCl2��Һ����СŨ��Ϊ ������֪Ksp��BaSO4����1��1��10-10��
��5���״���ˮ�ʻ����һ������Ⱦ����һ�ֵ绯ѧ��������������Ⱦ��ʵ��������ͼװ��ģ���������̣���ԭ���ǣ�ͨ���Co2+������Co3+��Ȼ����Co3+����������ˮ�еļ״�������CO2��������Co3+�Ļ�ԭ������Co2+����
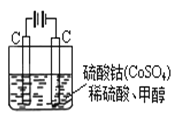
�� д�������缫��Ӧʽ�� ��
�� д����ȥˮ�еļ״������ӷ���ʽ�� ��
���𰸡���1��������1����HSO![]() �ĵ���̶�ǿ��ˮ��̶���1������ab��2����
�ĵ���̶�ǿ��ˮ��̶���1������ab��2����
��2��4��2������3��100L2/mol2��2��������1��������1����
��4��2��2��10-8mol/L��2����
��5����Co2+��e����Co3+��6Co3++CH3OH+H2O��CO2��+6Co2++6H+����2����
��������
�����������1�����������ɱ����е����ݿ�֪��HSO3-Խ�࣬����Խǿ����������������ӣ����뷽��ʽΪHSO3-H++SO32-����������������������ˮ����������Һ������ʱ������Ϊ�������ƺ����������ƣ�������ˮ��ij̶���ȣ���ϵ���غ���A��c��Na����+C��H+����2c��SO![]() ����c��HSO
����c��HSO![]() ��+C��OH-����C��H+��=C��OH-��,��c��Na������2c��SO
��+C��OH-����C��H+��=C��OH-��,��c��Na������2c��SO![]() ����c��HSO
����c��HSO![]() ����SO32-+H2OHSO3-+OH-��HSO3-H++SO32-������������ˮ�⣬������Ũ��Ϊc��Na+����c��HSO3-����c��SO32-����c��H+��=c��OH-����b��ȷ�� c��c��Na������c��H������c��SO
����SO32-+H2OHSO3-+OH-��HSO3-H++SO32-������������ˮ�⣬������Ũ��Ϊc��Na+����c��HSO3-����c��SO32-����c��H+��=c��OH-����b��ȷ�� c��c��Na������c��H������c��SO![]() ����c��HSO
����c��HSO![]() ����c��OH���������ϵ���غ㣬c������
����c��OH���������ϵ���غ㣬c������
��2��Na2SO3+HCl=NaCl+NaHSO3 NaHSO3+HCl=NaCl+H2O+SO2��
1 1 1 1 1 1 1
3mol X X X Y Y Y
X=3mol������Cl����HSO![]() �����ʵ���֮��Ϊ2��1���з��� ��X+Y��:��X-Y��=2:1 X:Y=3:1����X=3mol������ʽ��Y=1mol��������������HCl�����ʵ�������=X+Y=3+1=4mol��
�����ʵ���֮��Ϊ2��1���з��� ��X+Y��:��X-Y��=2:1 X:Y=3:1����X=3mol������ʽ��Y=1mol��������������HCl�����ʵ�������=X+Y=3+1=4mol��
��3�� CO��g��+ 2H2��g��![]() CH3OH��g����
CH3OH��g����
��ʼ 0��2mol 0��4mol 0
�仯 0.2��50% 0.4��50%
ƽ�� 0.1 0.2 0.1
����¶��µ�ƽ�ⳣ��Ϊ=0.1 /[��0.1/2�� ����0.2/2��2]= 100L2/mol2 ,���ַ�Ӧ�����CO��Ũ�ȣ���ת���ʼ��٣�����CO�����϶���ƽ�����ƣ�����COʣ��϶࣬���ɵ�CH3OH�����ȵ�һ��ƽ�����Զ�һЩ������CH3OH��ռ���������������٣��������������
��4����Һ��������,����ӱ���Ũ�ȼ��룬��BaCl2��ҺŨ��ΪC �������Ksp��BaSO4����1��1��10-10=C��Ba2+����C ��SO4��2-=0.02/2��C/2 C= 2��2��10-8mol/L
��5����ͨ�������Co2+������Co3+������������ʧ���ӷ���������Ӧ���缫��ӦΪCo2+-e-=Co3+������Co3+����������ˮ�еļ״�������CO2����������������ԭΪCo2+��ԭ���غ������غ��֪����ԭ����H+����ƽ��дΪ��6Co3++CH3OH+H2O=CO2��+6Co2++6H+��